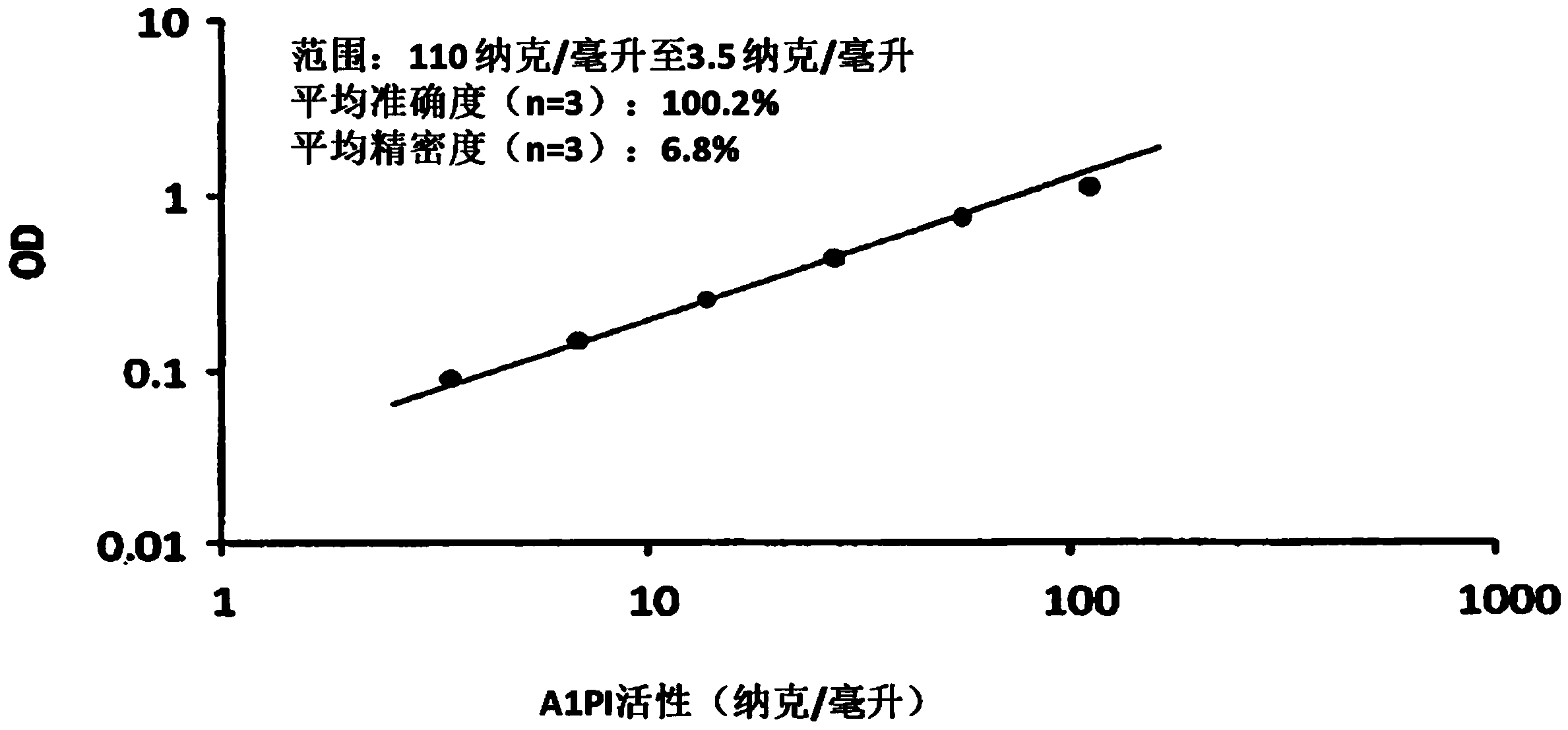
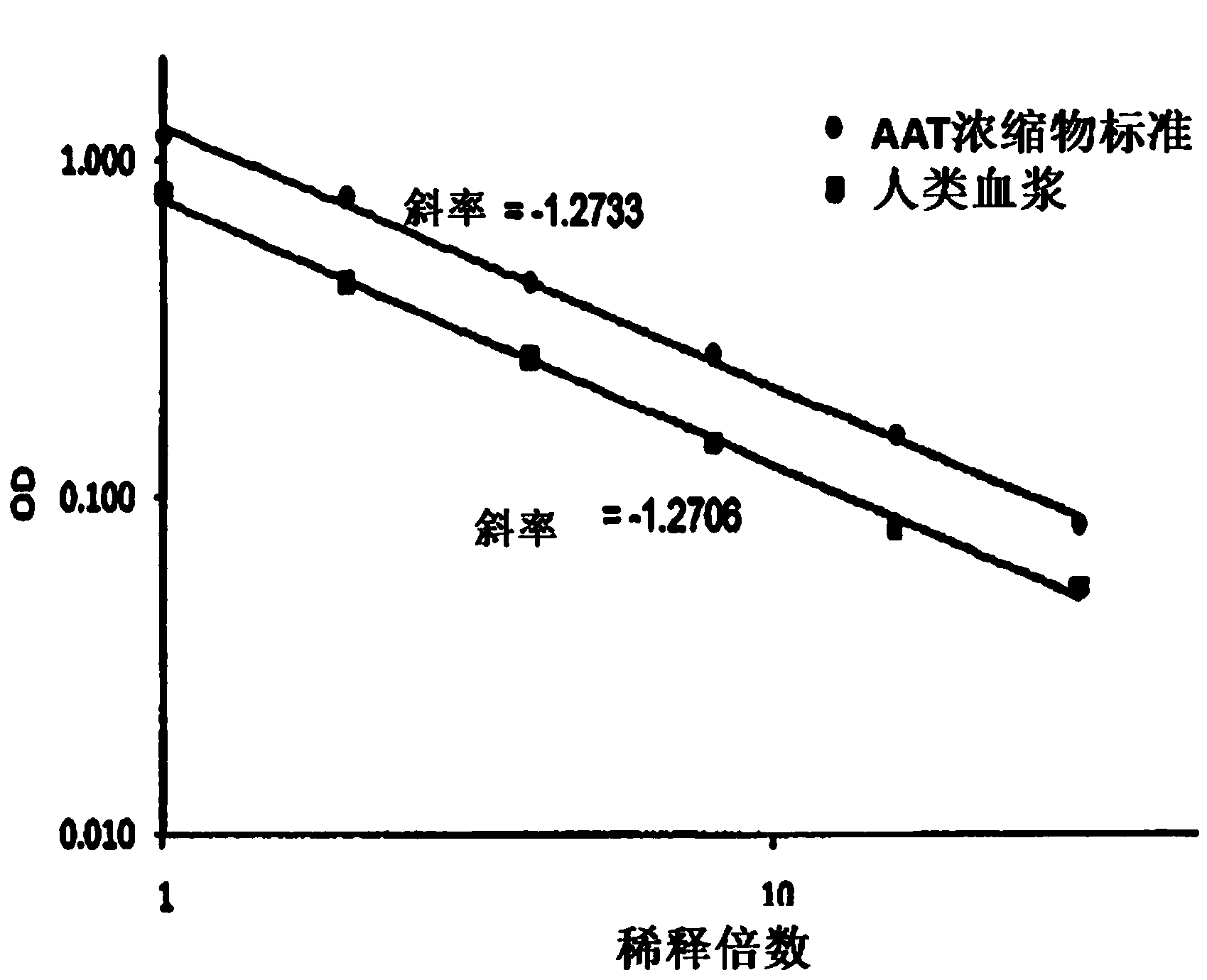
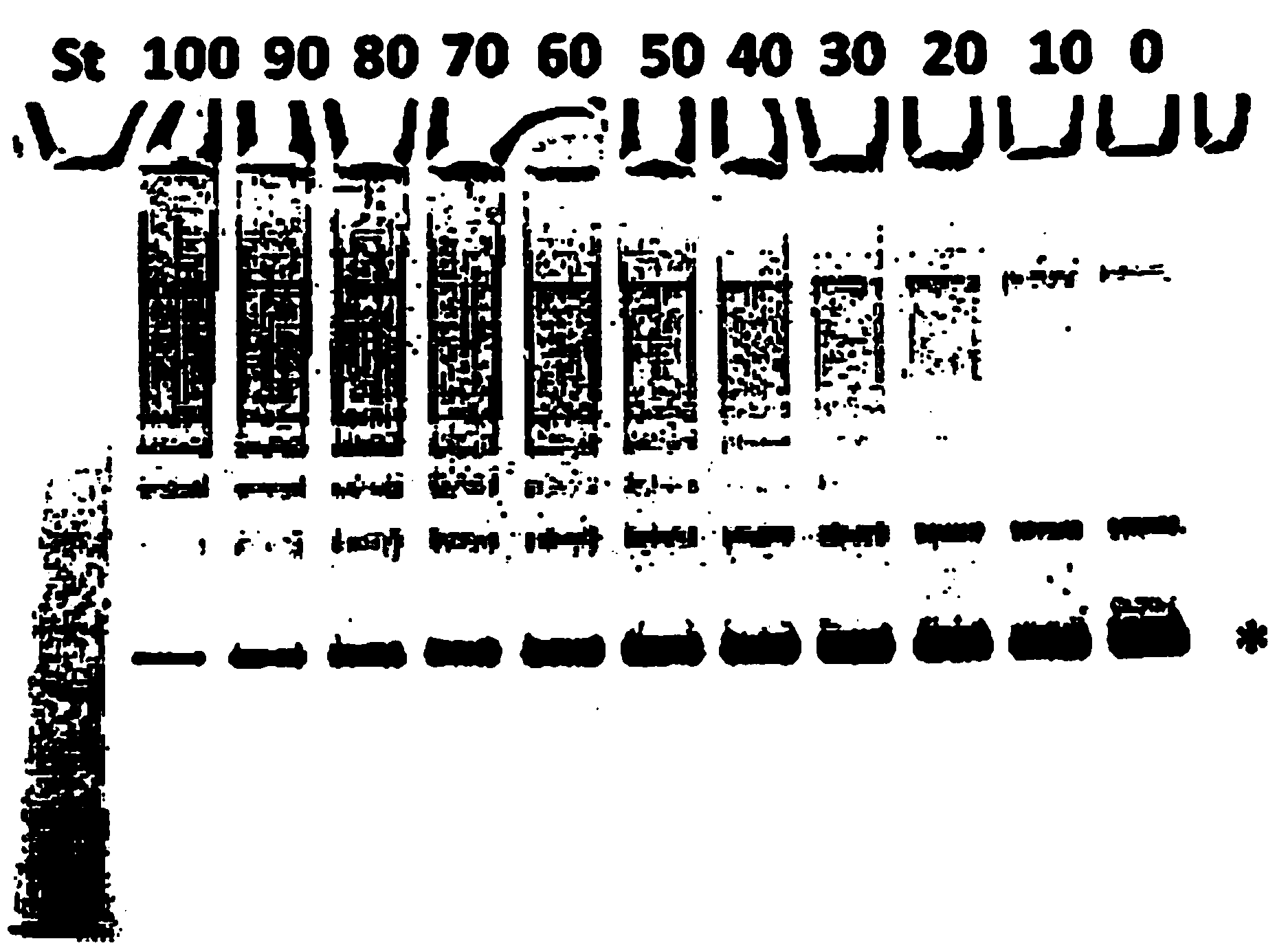
Solid phase-bound elastase-binding assay for the measurement of alpha1-antitrypsin activity
An elastase and protease inhibitor technology, which can be used in measurement devices, biological tests, and microbial determination/inspection, etc., which can solve the problem of quantitative limitation of active A1PI and the detection of excess reagents, such as elastase, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0007] In one aspect, the present invention relates to a method for measuring activity alpha in a sample 1 - A method for protease inhibitors (A1PI), comprising the steps of:
[0008] (a) binding elastase to a solid phase;
[0009] (b) incubating the solid phase with the sample;
[0010] (c) incubating the solid phase with the detection reagent bound to A1PI;
[0011] (d) determining the amount of detection reagent bound to the solid phase; and
[0012] (e) Determining the amount of active AlPI in the sample.
[0013] In this context, the term "measuring active A1PI in a sample" as used herein refers to determining the amount and / or concentration of active A1PI contained in a sample. Further, the term "active AlPI" as used herein refers to AlPI capable of displaying its natural function (ie, capable of binding and inactivating elastase and other serine proteases).
[0014] The elastase used in the method of the present invention is not particularly limited, and may be, fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com