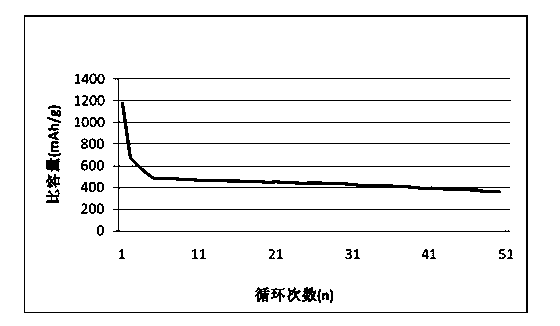
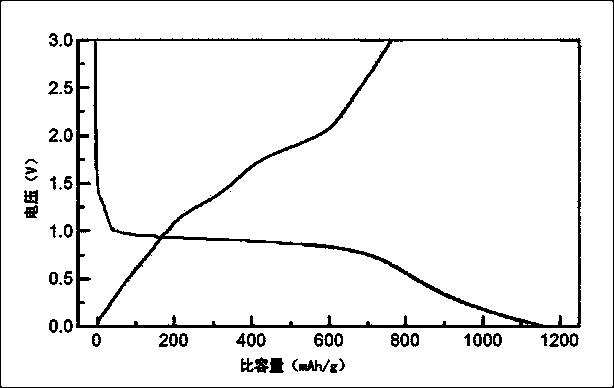
Preparation method of calcium-cobalt oxide compound as anode material for lithium ion batteries
A lithium-ion battery and anode material technology, which is applied in the field of lithium-ion battery anode materials and its preparation, can solve problems such as the adverse effects of lithium-ion battery materials, and achieve the effects of small and uniform size, high specific capacity, and excellent cycle performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Weigh 0.75mol Ca(CH 3 COO) 2 and 1mol Co(CH 3 COO) 2 , Weigh 2 mol of chelate citric acid, put the aforementioned reaction metal salt and chelate into a reaction vessel, add 4L of deionized water to dissolve the reactants. The mixed solution was stirred to a sol state under the stirring state, the stirring temperature was controlled at 80° C., and the stirring time was 5 h; after the stirring was completed, the sol was dried in an oven at 100° C. for 10 h to obtain a xerogel. The dry gel is pulverized and sintered in a high-temperature furnace. Pre-fire at 400°C for 24 hours, then heat up to 600°C and sinter for 4.5 hours to obtain Ca 3 co 4 o 9 finished product.
[0025] Will make Ca 3 co 4 o 9 The finished product is mixed with the conductive agent SuperP carbon black and the binder PVDF at a ratio of 70:20:10, and the organic solvent 1-methyl-2-pyrrolidone (NMP) is added as a solvent for stirring. After stirring evenly with a high-speed mixer, it is coated...
Embodiment 2
[0027] Weigh 0.375mol Ca(CH 3 COO) 2 and 0.5mol Co(CH 3 COO) 2 , Weigh 1 mol of chelate tartaric acid, put the reaction metal salt and chelate into a reaction vessel, add 1.5L deionized water to dissolve the reactants. The mixed solution was stirred to a sol state under the stirring state, the stirring temperature was controlled at 90° C., and the stirring time was 6 hours; after the stirring was completed, the sol was dried in an oven at 100° C. for 14 hours to obtain a xerogel. The dry gel is pulverized and sintered in a high-temperature furnace. Pre-fire at 450°C for 16 hours, then heat up to 700°C for 3 hours to obtain Ca 3 co 4 o 9 finished product.
[0028] Will make Ca 3 co 4 o 9 The finished product is mixed with the conductive agent SuperP carbon black and the binder PVDF at a ratio of 70:20:10, and the organic solvent 1-methyl-2-pyrrolidone (NMP) is added as a solvent for stirring. After stirring evenly with a high-speed mixer, it is coated on the surface ...
Embodiment 3
[0030] Weigh 0.375mol Ca(CH 3 COO) 2 and 0.5mol Co(CH 3 COO) 2 , Weigh 3 mol of the chelate gluconic acid, put the reaction metal salt and the chelate into a reaction vessel, and add 8L of deionized water to dissolve the reactant. The mixed solution was stirred to a sol state under the stirring state, the stirring temperature was controlled at 60° C., and the stirring time was 6 hours; after the stirring was completed, the sol was dried in an oven at 100° C. for 16 hours to obtain a xerogel. The dry gel is pulverized and sintered in a high-temperature furnace. Pre-fire at 350°C for 24 hours, then heat up to 650°C and sinter for 6 hours to obtain Ca 3 co 4 o 9 finished product.
[0031] Will make Ca 3 co 4 o 9 The finished product is mixed with the conductive agent SuperP carbon black and the binder PVDF at a ratio of 70:20:10, and the organic solvent 1-methyl-2-pyrrolidone (NMP) is added as a solvent for stirring. After stirring evenly with a high-speed mixer, it is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com