Nonaqueous electrolyte secondary battery
A non-aqueous electrolyte and secondary battery technology, which is applied in secondary batteries, secondary battery charging/discharging, secondary battery repair/maintenance, etc. It can solve the problems of high cobalt price and low resource existence, and achieve the purpose of suppressing battery life. Increased thickness, suppressed side reactions, and improved capacity retention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
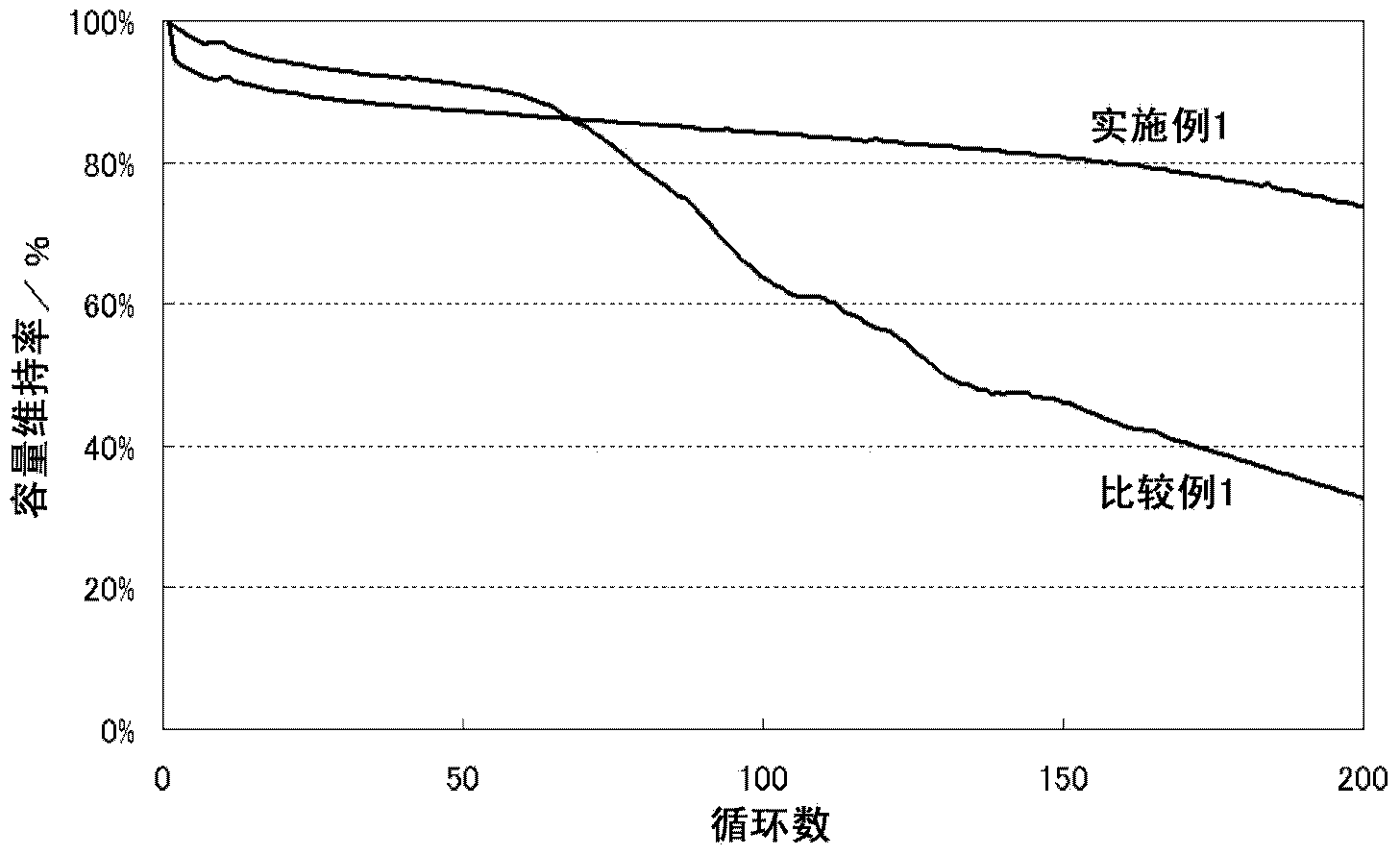
Examples
Embodiment 1
[0046] [Positive electrode active material]
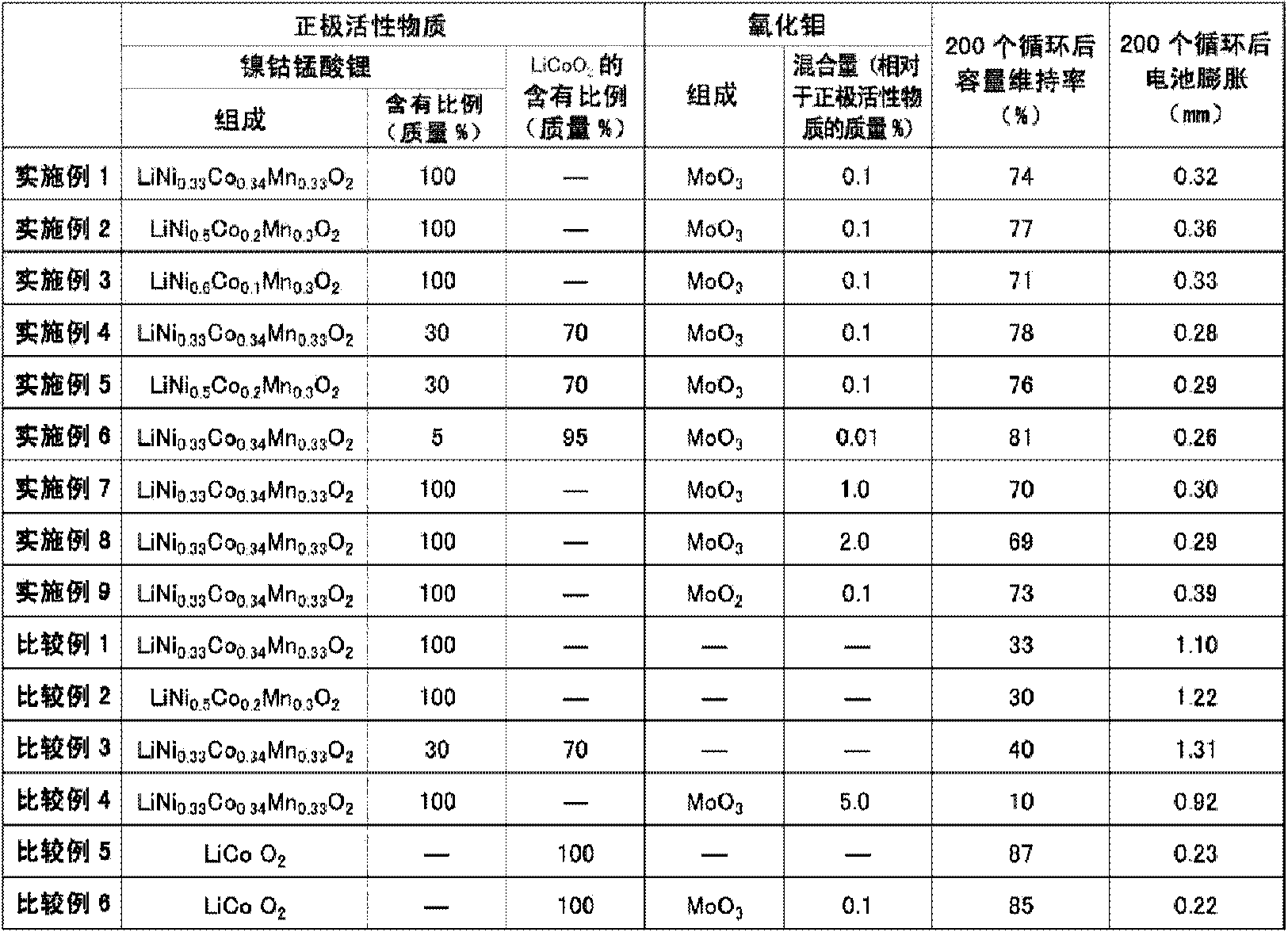
[0047] Lithium nickel cobalt manganate as a positive electrode active material was obtained as follows. As a starting material, lithium hydroxide (LiOH·H 2 O). Co-precipitated hydroxides of nickel, cobalt, and manganese (Ni 0.33 co 0.34 mn 0.33 (OH) 2 ). These were weighed and mixed so that the molar ratio of lithium and a transition metal (nickel, cobalt, and manganese) might become 1:1. The obtained mixture was calcined at 400° C. for 12 hours in an oxygen atmosphere, crushed with a mortar, and then calcined at 900° C. for 24 hours in an oxygen atmosphere to obtain lithium nickel cobalt manganese oxide. This was pulverized with a mortar to an average particle diameter of 15 μm to prepare the positive electrode active material used in this example. Furthermore, the chemical composition of lithium nickel cobalt manganese oxide was measured by ICP (Inductively Coupled Plasma: Inductively Coupled Plasma Luminescence Analysis)...
Embodiment 2 and 3
[0063] In Examples 2 and 3, a nonaqueous electrolyte secondary battery was produced in the same manner as in Example 1 except that the composition ratio of nickel, cobalt, and manganese in nickel-cobalt lithium manganese oxide was changed.
Embodiment 4~6
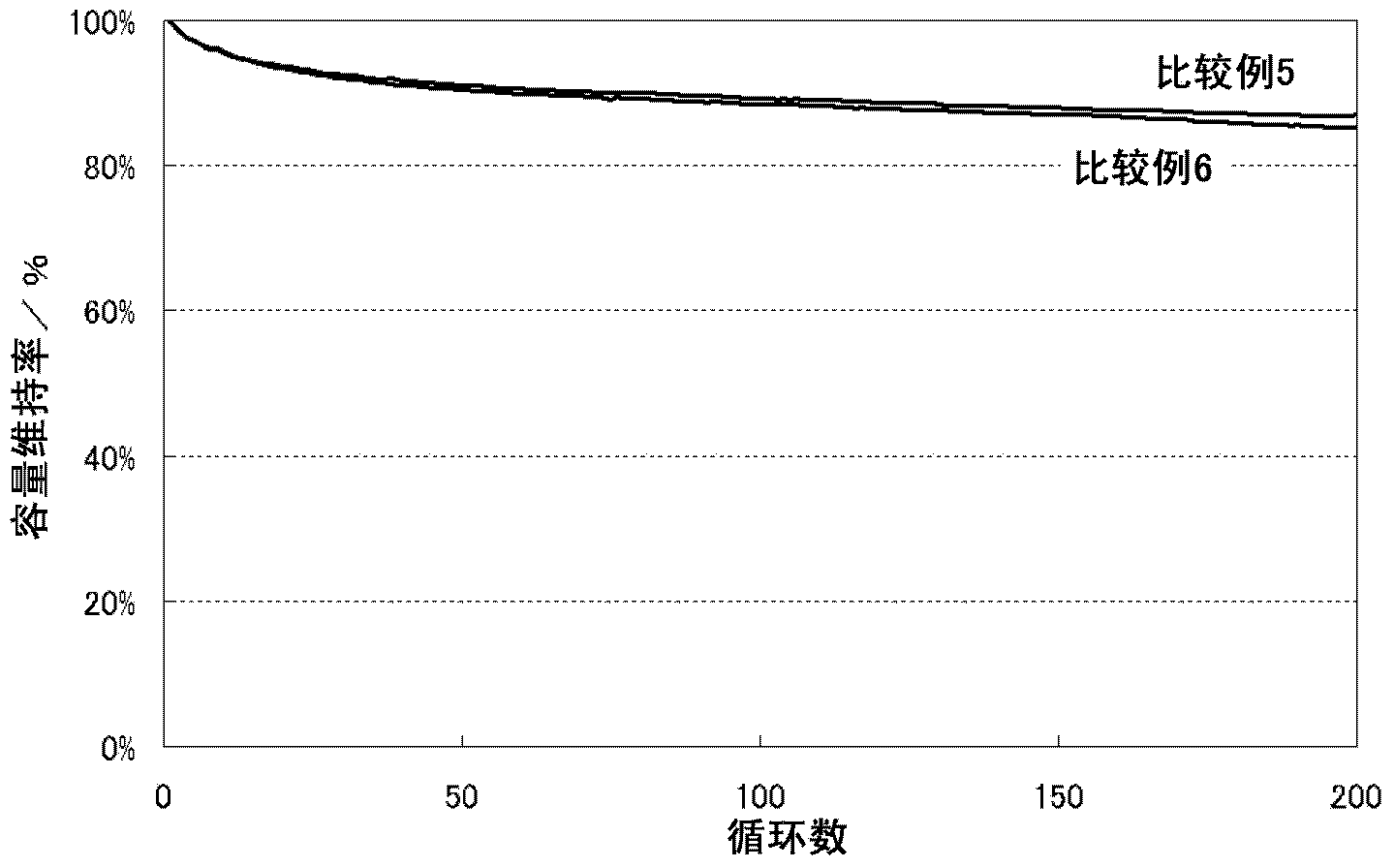
[0065] In Examples 4 to 6, a mixture obtained by mixing nickel-cobalt lithium manganese oxide and lithium cobalt oxide used in Example 1 or 2 at a given mixing ratio was used as the positive electrode active material, and, in Example 6 , the MoO 3 A non-aqueous electrolyte secondary battery was fabricated in the same manner as in Example 1 except that the blending amount of the mixture was changed to 0.01% by mass relative to the positive electrode active material.
[0066] Lithium cobaltate as a positive electrode active material was obtained as follows. As a starting material, lithium carbonate (Li 2 CO 3 ). As the cobalt source, tricobalt tetroxide (Co 3 o 4 ). Weigh them and mix them in a mortar so that the molar ratio of lithium to cobalt is 1:1. The resulting mixture was fired at 850° C. for 20 hours in an air atmosphere to obtain lithium cobaltate. The positive electrode active material was produced by pulverizing this in a mortar to an average particle diameter o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com