Luminescent solar concentrator comprising disubstituted benzoselenadiazole compounds
A technology of benzoselenodiazole and solar energy, which is applied in the direction of luminescent materials, chemical instruments and methods, photovoltaic power generation, etc., and can solve the problems of little absorption and inability to convert photovoltaic cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
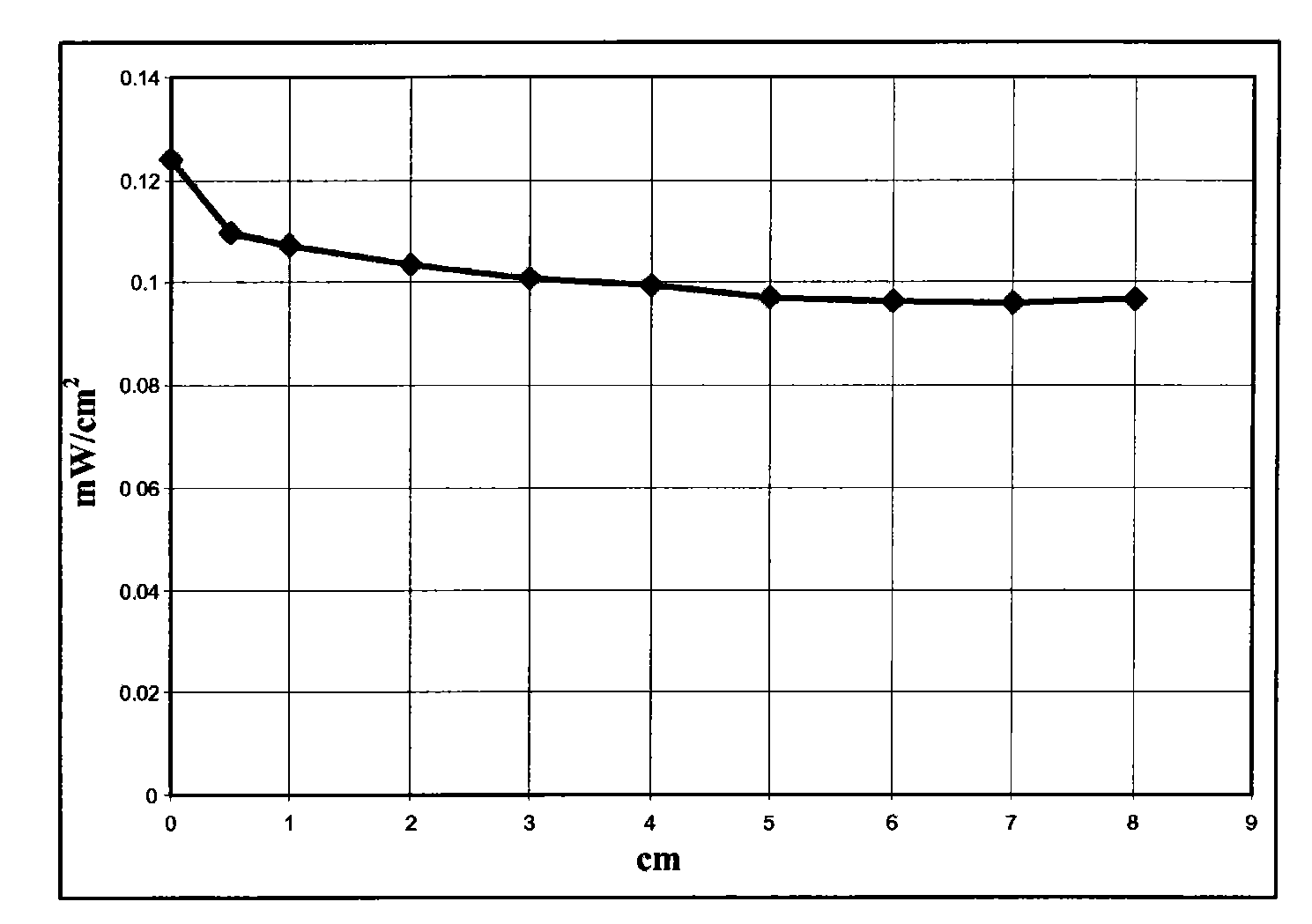
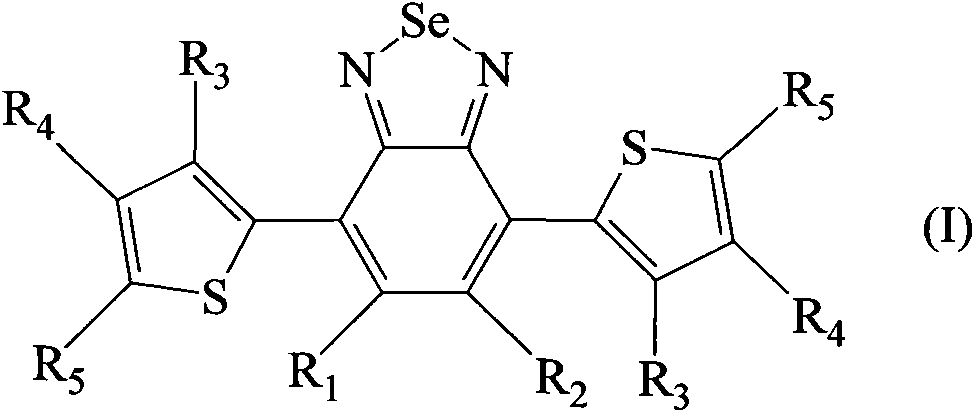
[0058] Dissolve 6 g of polymethyl methacrylate Altuglas VSUVT100 (PMMA) and 57.2 mg of 4,7-di-(thiophen-2'-yl)-2,1,3-benzoselenadiazole in 30 ml of 1,2 -in dichlorobenzene. The obtained solution was uniformly deposited on a sheet (90×90×6 mm) of polymethylmethacrylate Altuglas VSUVT100 (PMMA) using a medical blade film coater, and the solvent was heated at room temperature (25° C.) at Evaporate for 24 hours in a small air stream. A red transparent tablet (sheet 1) was obtained, the color being imparted by a film whose thickness proved to be in the range of 300 μm to 350 μm.
[0059] Then set the surface area to 1.2cm 2 The photovoltaic cell IXYS-XOD17 was applied to one edge of the polymer sheet.
[0060] Then use a power of 1sun (1000W / m 2 ) light source illuminates the main side of the polymer sheet (covered by a thin film containing 4,7-bis-(thiophen-2'-yl)-2,1,3-benzoselenadiazole) and measures the Electric power.
[0061] The power measurement is achieved by coverin...
example 2
[0064] Example 2 (comparison)
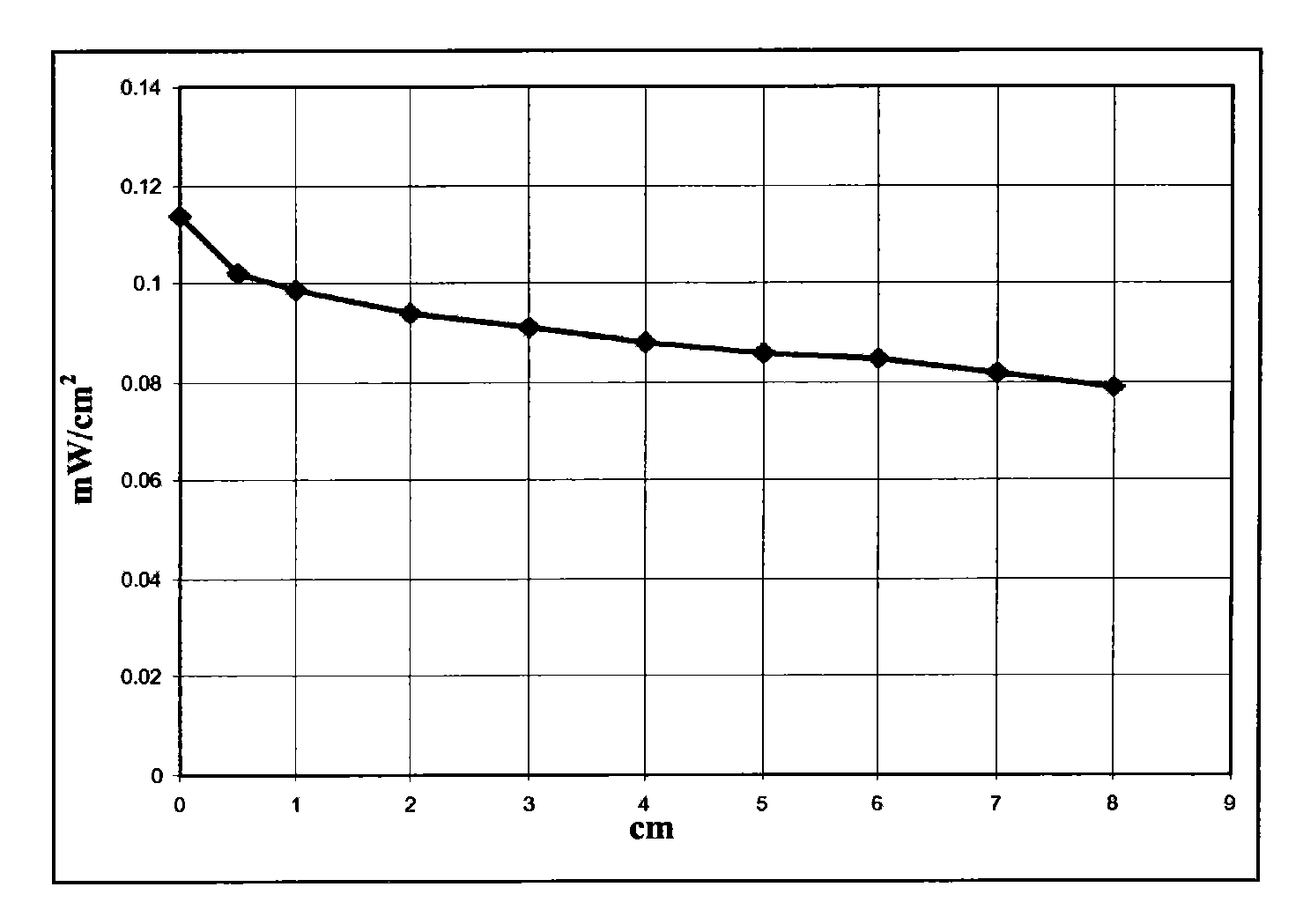
[0065] Dissolve 6g of polymethylmethacrylate Altuglas VSUVT100 (PMMA) and 49.5mg of 4,7-di-(thiophen-2'-yl)-2,1,3-benzothiadiazole (DTB) in 30ml of 1,2-Dichlorobenzene. Then, the obtained solution was uniformly deposited on a sheet (size 90×90×6 mm) of polymethyl methacrylate Altuglas VSUUT100 (PMMA) using a medical blade film coater, and the solvent was heated at room temperature (25° C.) , Evaporate for 24 hours in a small air stream. A red transparent tablet (sheet 2) was obtained, the color being imparted by a film whose thickness proved to be in the range of 300 μm to 350 μm.
[0066] Then set the surface area to 1.2cm 2 The photovoltaic cell IXYS-XOD17 was applied to one edge of the polymer sheet.
[0067] Then use a power of 1sun (1000W / m 2 ) light source illuminates the main side of the polymer sheet (covered by a film containing 4,7-bis-(thiophen-2'-yl)-2,1,3-benzothiadiazole) and measures the effect by illumination generated el...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com