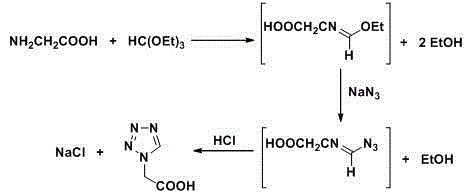
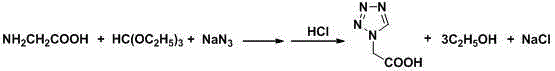Post-treatment method for preparing 1h-tetrazolium acetic acid from triethyl orthoformate
A technology of triethyl orthoformate and tetrazolium acetic acid, applied in the field of organic synthesis, can solve problems such as separation problems, low recovery rate of organic solvents, large amount of ethyl acetate, etc., and achieve enhanced simplicity and repeatability , Facilitate industrial production and reduce production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In a 2 L three-necked flask, add 286 ml (255 g, 1.7 mol) of triethyl orthoformate, 78 g (1.2 mol) of sodium azide and 500 ml of acetic acid, stir at 65 °C until the sodium azide is completely dissolved, batchwise Add 90 grams of glycine (1.2 mol, add in 4 hours, 1.9 g / 5 min), and continue stirring at 65 °C for 2 hours after the addition, to obtain a reaction liquid; after the reaction, raise the temperature and control the distillation temperature to 76-82 °C, and recover the side The product ethanol was 175 ml; then, the temperature was raised to control the distillation temperature to 115-120 ° C, and 480 ml of acetic acid was recovered as a solvent; after the organic solvent was recovered by distillation, 940 ml of 36% hydrochloric acid was added to the residue, stirred at room temperature for 1 h, and the chlorinated acid was removed by filtration. 67 grams of sodium (theoretical value 70.2 grams); add 3 grams of activated carbon in the filtrate, reflux and stir for ...
Embodiment 2
[0026] In a 2 L three-necked flask, add 286 ml (255 g, 1.7 mol) of triethyl orthoformate, 78 g (1.2 mol) of sodium azide and 500 ml of acetic acid, stir at 65 °C until the sodium azide is completely dissolved, batchwise Add 90 g of glycine (1.2 mol, added in 4 hours, 1.875 g / 5 min), and continue to stir at 65 °C for 2 hours after the addition, to obtain a reaction liquid; after the reaction, raise the temperature and control the distillation temperature to 76-82 °C, and recover the side The product ethanol was 180 ml; then, the temperature was raised to control the distillation temperature to 115-120 ° C, and 482 ml of acetic acid was recovered as a solvent; after the organic solvent was recovered by distillation, 520 ml of 36% hydrochloric acid was added to the residue, stirred at room temperature for 1 h, and the chlorinated acid was removed by filtration. 68 grams of sodium (theoretical value 70.2 grams); 3 grams of activated carbon was added to the filtrate, stirred under r...
Embodiment 3
[0028] In a 2 L three-necked flask, add 286 ml (255 g, 1.7 mol) of triethyl orthoformate, 78 g (1.2 mol) of sodium azide and 500 ml of acetic acid, stir at 65 °C until the sodium azide is completely dissolved, batchwise Add 90 g of glycine (1.2 mol, added in 4 hours, 1.875 g / 5 min), and continue to stir at 65 °C for 2 hours after the addition, to obtain a reaction liquid; after the reaction, raise the temperature and control the distillation temperature to 76-82 °C, and recover the side The product ethanol was 180 ml; then, the temperature was raised to control the distillation temperature to 115-120 ° C, and 483 ml of acetic acid was recovered as a solvent; after the organic solvent was recovered by distillation, 310 ml of 36% hydrochloric acid was added to the residue, stirred at room temperature for 1 h, and the chlorinated acid was removed by filtration. 70 grams of sodium (theoretical value 70.2 grams); 3 grams of activated carbon was added to the filtrate, stirred under r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com