A kind of carbon dioxide electrochemical reduction catalyst and its preparation and application
A carbon dioxide and electrochemical technology, which is applied in the field of carbon dioxide electrochemical reduction catalyst and its preparation and application, can solve the problems that the electrochemical reduction effect has not received widespread attention, so as to increase the catalytic activity of electrochemical reduction, have a good application prospect, reduce the The effect of the hydrogen evolution reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
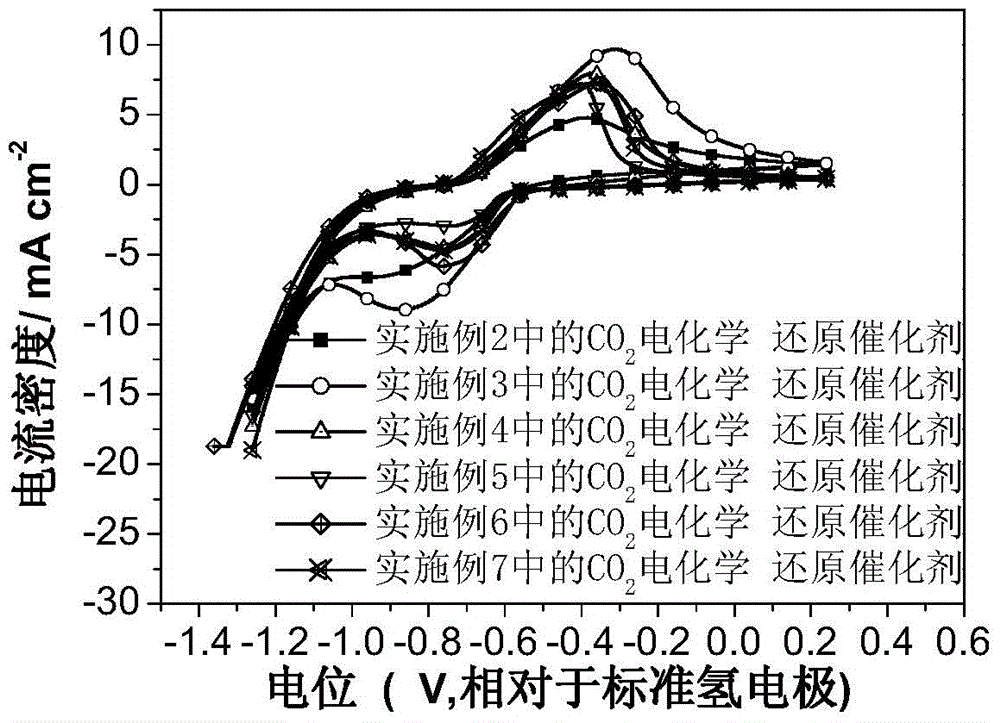
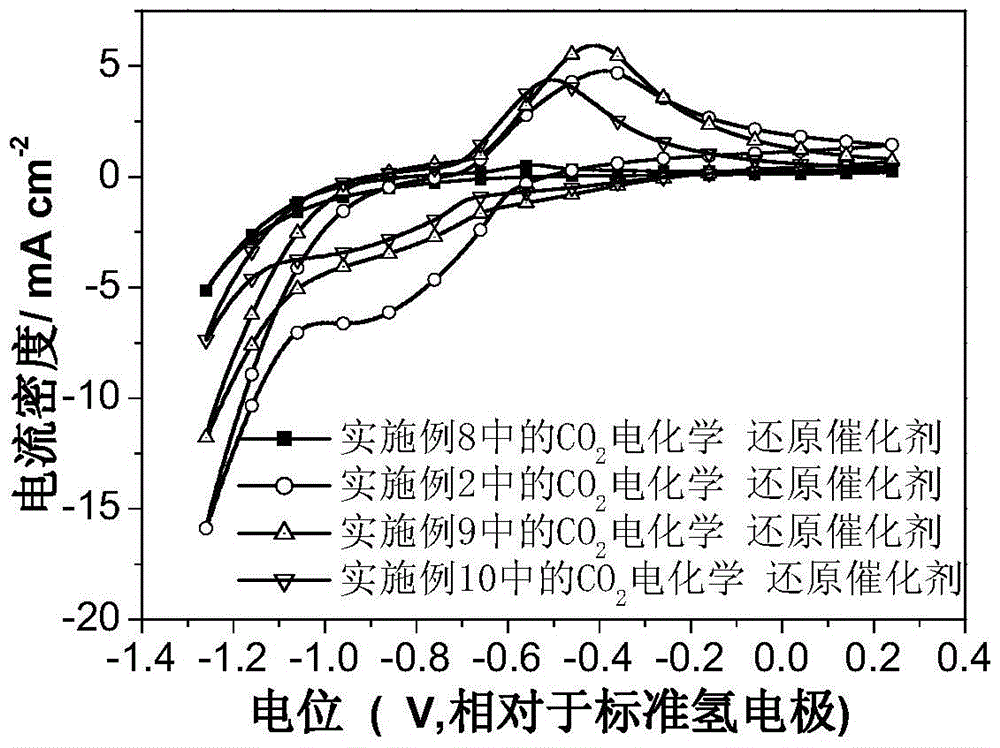
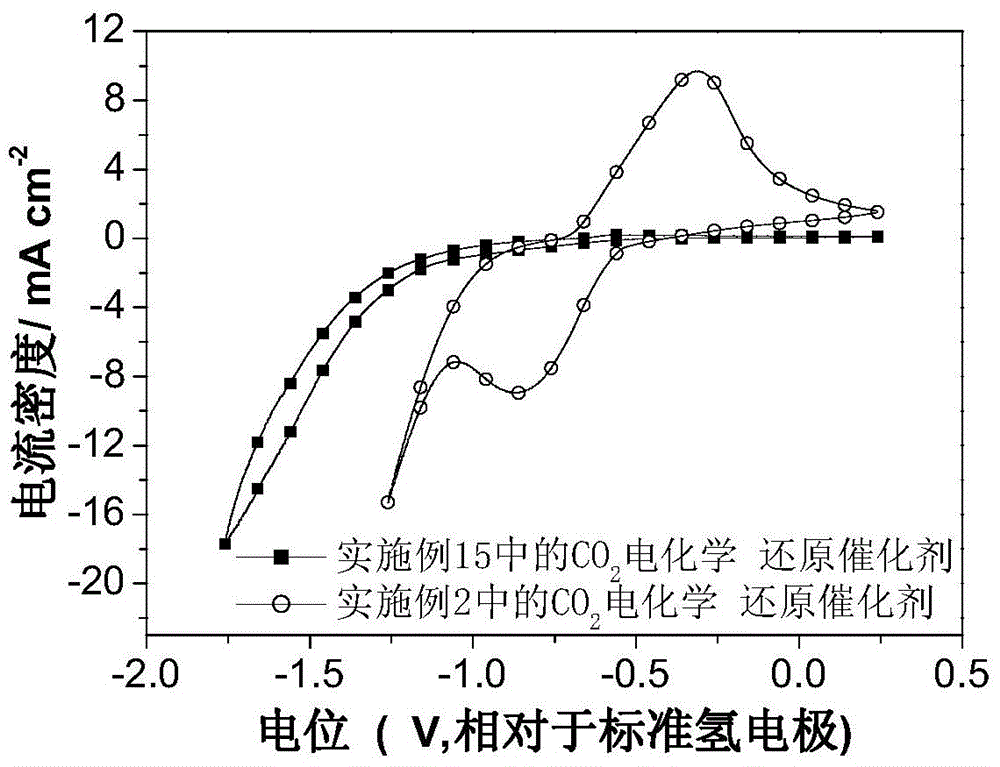
Image
Examples
Embodiment 1
[0035] SnO 2 Preparation of nanoflower catalyst precursor: Dissolve 0.004mol stannous chloride dihydrate and 0.01mol trisodium citrate in 10mL deionized water to form a mixture of 0.4M stannous chloride and 1M trisodium citrate Solution, dissolve 0.002mol sodium hydroxide in 10ml of absolute ethanol, take 10mL of the above-mentioned 0.4M stannous chloride and 1M trisodium citrate mixed solution and 10ml 0.2M sodium hydroxide solution and fully mix to obtain SnO 2 Nanoflower catalyst precursor, and moved into a 100mL reactor.
Embodiment 2
[0037] An electrochemical reduction catalyst for carbon dioxide, comprising tin dioxide nanoflowers, the tin dioxide nanoflowers are synthesized by hydrothermal reaction, and the preparation method is as follows: take 0.004mol of stannous chloride dihydrate and 0.01mol of triscitric acid Dissolve sodium together in 10mL deionized water to make a mixed solution of 0.4M stannous chloride and 1M trisodium citrate, dissolve 0.002mol sodium hydroxide in 10ml absolute ethanol, take 10mL of the above 0.4M chlorine The mixed solution of stannous chloride and 1M trisodium citrate and 10ml 0.2M sodium hydroxide solution are fully mixed to obtain SnO 2 Nanoflower catalyst precursor, and moved into a 100mL reactor. The reaction kettle is a hydrothermal reaction kettle with a polytetrafluoroethylene liner and a stainless steel jacket. Put the reaction kettle in an oven at 180°C for a hydrothermal reaction for 2 hours, centrifuge, wash with ethanol, and dry to obtain tin dioxide Nanoflower...
Embodiment 3
[0039] An electrochemical reduction catalyst for carbon dioxide, comprising tin dioxide nanoflowers, the tin dioxide nanoflowers are synthesized by hydrothermal reaction, and the preparation method is as follows: take 0.004mol of stannous chloride dihydrate and 0.01mol of triscitric acid Dissolve sodium together in 10mL deionized water to make a mixed solution of 0.4M stannous chloride and 1M trisodium citrate, dissolve 0.002mol sodium hydroxide in 10ml absolute ethanol, take 10mL of the above 0.4M chlorine The mixed solution of stannous chloride and 1M trisodium citrate and 10ml 0.2M sodium hydroxide solution are fully mixed to obtain SnO 2 Nanoflower catalyst precursor, and moved into a 100mL reactor. The reaction kettle is a hydrothermal reaction kettle with a polytetrafluoroethylene liner and a stainless steel jacket. Put the reaction kettle into an oven at 180°C for a hydrothermal reaction for 5 hours, centrifuge, wash with ethanol, and dry to obtain tin dioxide Nanoflow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com