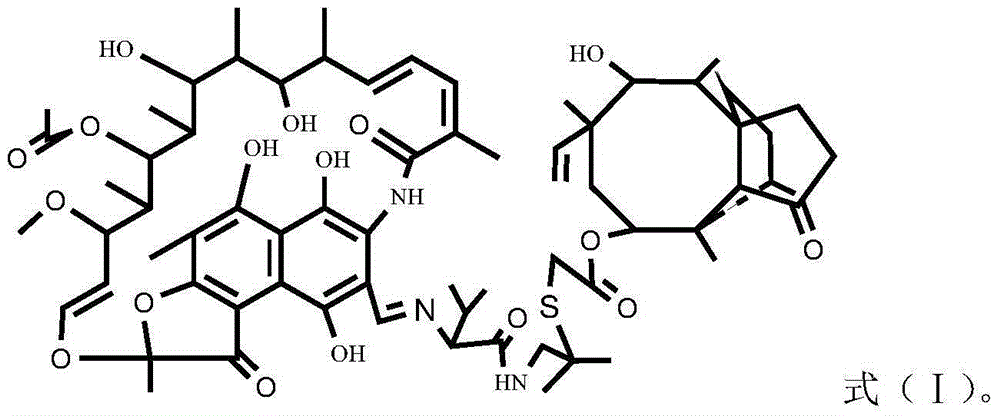
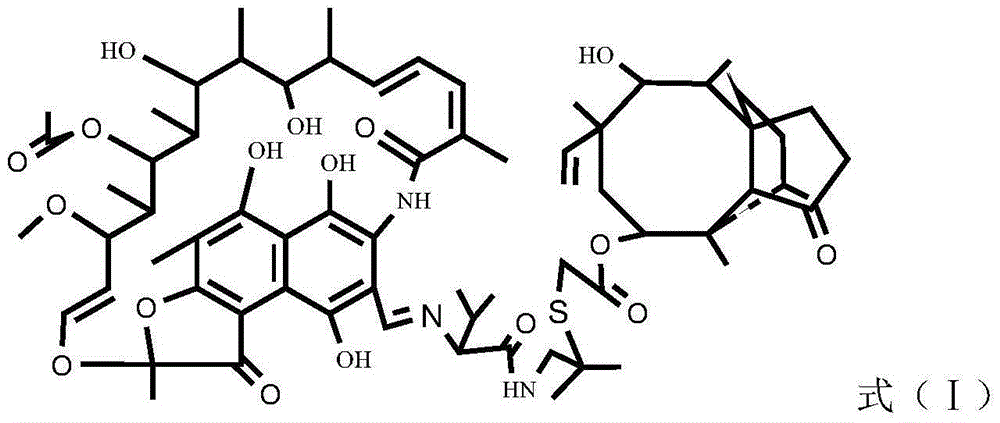
Rifamycin-like vonimulin hybrid antibiotic and preparation method thereof
A technology of rifamycin and warnimulin, which is applied in the field of chemical synthesis of drugs, can solve the problems of insufficient ester bond and short half-life, and achieve good therapeutic effect, enhanced antibacterial effect and stable linking group
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
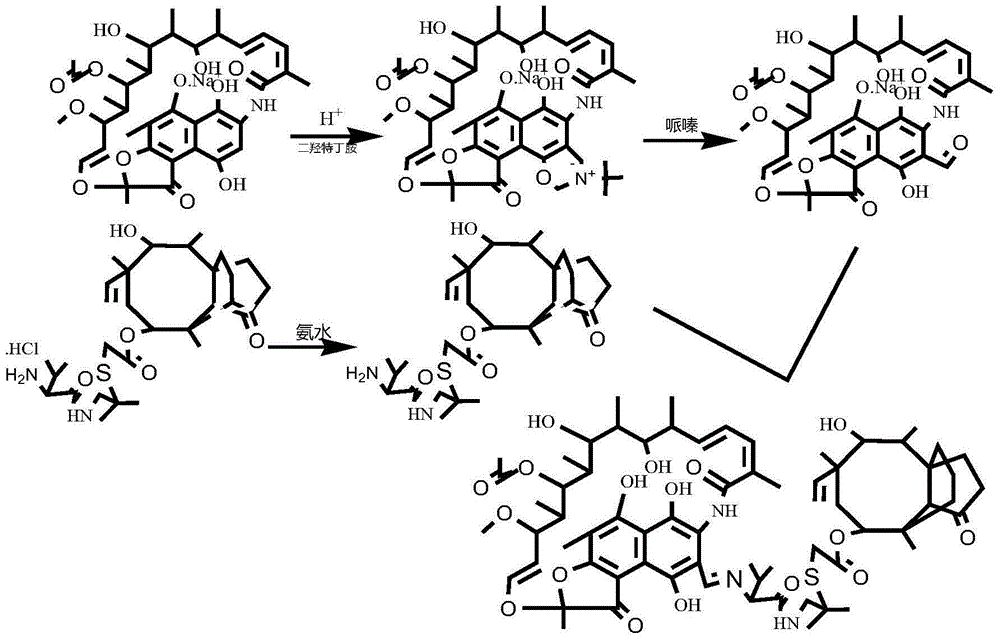
[0023] The preparation process of the rifamycin class warnemulin hybrid antibiotic is as follows:
[0024] 1) Dissolve rifamycin s-sodium salt in the solvent DMF and mix evenly, add concentrated sulfuric acid to acidify for 0.5h by mole rifamycin s-sodium salt:concentrated sulfuric acid=1.9~2:1, then add dihydroxy Terbutylamine, dihydroxyterbutylamine and rifamycin S-sodium salt with a molar ratio of 1.5 to 1.9:1, heated to 35 to 50°C, kept warm for 2 hours, cooled, added a precipitant to stand still, filtered and washed with water to obtain Intermediate oxazine rifamycin Ⅱ;
[0025] 2) Mix the intermediate oxazine rifamycin II and anhydrous piperazine in a protic solvent at a molar ratio of 1:1.2-1.5, react at 45-60°C for 2 hours, and obtain the intermediate 3-formazine by column chromatography Acyl rifamycin III;
[0026] 3) Add warnemulin hydrochloride into water to dissolve completely, adjust the pH value to 8-9 with an acid-binding agent, filter, wash with water, and dr...
Embodiment 1
[0034] According to the above steps, the specific process and parameters are determined as follows:
[0035] In step 1), acidify by mole bifamycin s-sodium salt: concentrated sulfuric acid=1.9:1; molar ratio dihydroxyterbumine: rifamycin s-sodium salt=1.5:1 feed; wherein, rifampicin The concentration of mycin s-sodium salt in the solvent DMF was 0.02 g / mL.
[0036] In step 2), the intermediate oxazine rifamycin II: anhydrous piperazine is added according to the molar ratio of 1:1.2. The protic solvent is ethanol.
[0037] Step 3) in, acid binding agent selects saturated NaHCO for use 3 solution.
[0038] In step 4), the intermediate 3-formyl rifamycin III: the intermediate vornimulin IV is added according to the molar ratio of 1:1.1; the intermediate 3-formyl rifamycin III is dissolved in the solvent tetrahydrofuran at a concentration of 0.025g / mL.
[0039]The target product I can be obtained. Dissolve it in 1.2% ethanol aqueous solution to prepare a 0.5 mg / L solution, p...
Embodiment 2
[0041] According to the above steps, the specific process and parameters are determined as follows:
[0042] In step 1), acidify by mole bifamycin s-sodium salt: concentrated sulfuric acid=2:1; molar ratio dihydroxyterbumine: rifamycin s-sodium salt=1.9:1 feed; wherein, rifampicin The concentration of mycin s-sodium salt in the solvent DMF was 0.05 g / mL.
[0043] In step 2), the intermediate oxazine rifamycin II: anhydrous piperazine is added according to the molar ratio of 1:1.2. The protic solvent is ethanol.
[0044] Step 3) in, acid binding agent selects saturated NaHCO for use 3 solution.
[0045] In step 4), the intermediate 3-formyl rifamycin III: the intermediate vornimulin IV is added according to the molar ratio of 1:1.1; the intermediate 3-formyl rifamycin III is dissolved in the solvent tetrahydrofuran at a concentration of 0.0725g / mL.
[0046] The target product I can be obtained. Dissolve it in 1.2% ethanol aqueous solution to prepare a 0.5 mg / L solution, p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com