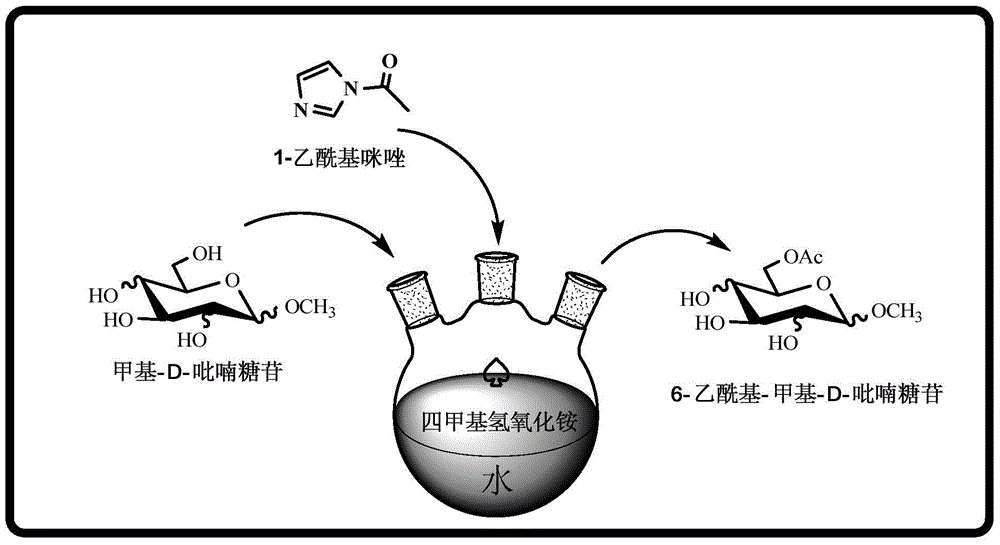
A kind of synthetic method of regioselective acetylated methyl-d-pyranoside
An acetylated methyl group and regioselectivity technology, applied in the fields of fine chemicals and sugar chemical synthesis, can solve problems such as environmental pollution and complicated operation, and achieve the effects of reducing reaction toxicity, low cost and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
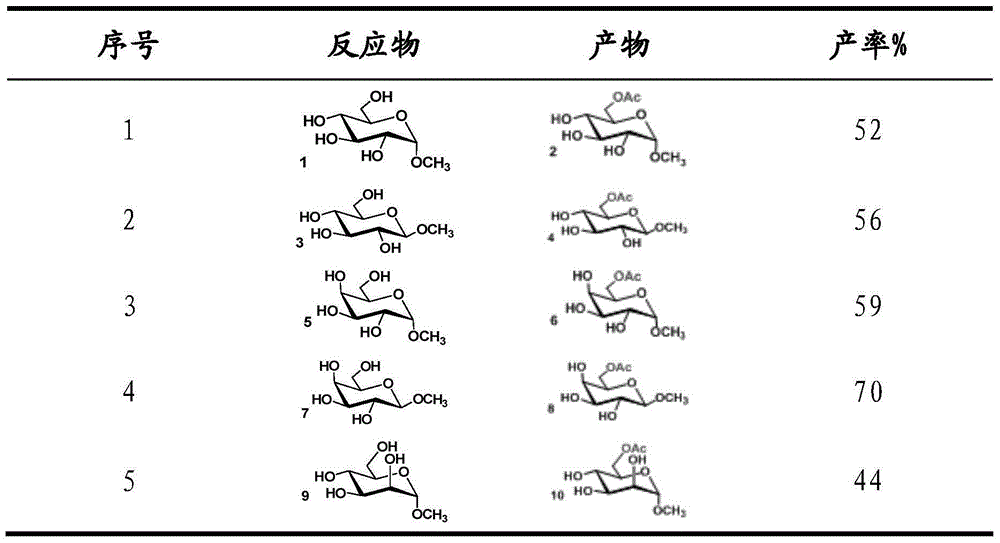
[0020] At 60°C, use 200 μL of water as a solvent, mix 50 mg (1.0 eq) of methyl-α-D-glucopyranoside and 114 μL (1.2 eq) of 25% tetramethylammonium hydroxide solution, and then add 1 - Acetyl imidazole 85.9mg (3.0eq), after 18 hours of reaction, the solvent was removed under reduced pressure, and the 6-position acetylated methyl-α-D-glucopyranoside derivative was obtained by column separation, and the column separation yield was 52%.
Embodiment 2
[0022] At 60°C, use 200 μL of water as a solvent, mix 50 mg (1.0 eq) of methyl-β-D-glucopyranoside and 114 μL (1.2 eq) of 25% tetramethylammonium hydroxide solution, and then add 1 -Acetylimidazole 85.9mg (3.0eq), after reacting for 14h, the solvent was removed under reduced pressure, and the 6-position acetylated methyl-β-D-glucopyranoside derivative was obtained by column separation, and the column separation yield was 56%.
Embodiment 3
[0024] At 60°C, use 200 μL of water as a solvent, add 50 mg (1.0 eq) of methyl-α-D-galactopyranoside and 114 μL (1.2 eq) of tetramethylammonium hydroxide (25% aqueous solution), Then add 85.9 mg (3.0 eq) of 1-acetylimidazole, react for 18 hours, remove the solvent under reduced pressure, and obtain 6-acetylated methyl-α-D-galactopyranoside derivatives through column separation. rate 59%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com