A kind of biofilm device for wound surface and preparation method thereof
A biofilm and wound technology, applied in the field of medical materials, can solve the problems of high cost of treatment, pain of patients, complex artificial skin, etc., and achieve the effect of simple and easy-to-use equipment, less labor, and uncomplicated preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: to the therapeutic effect of scald and pathological examination result
[0038]Take 36 mice with a body weight of 20-24g, and divide them into 3 groups randomly, 12 mice / group; depilate the back with 10% sodium sulfide, and use 80°C water to cause II degree burns with an area of 1.4×1.4cm. The first group of mice used the biofilm device as the experimental group; the second group of mice used Jingwanhong scald medicine as the positive control group, and the third group of mice used everything as the blank control group.
[0039] The results showed that: 1. The experimental group and the positive control group began to grow scabs on the second day. After 4-7 days, the scabs on the skin of all mice came off, and the new granulation was ruddy, and hairs gradually grew. 2. The positive control group (i.e. the first group) is slower than the experimental group (i.e. the second group); 3. The blank control group (i.e. the third group) is at the initial stage o...
Embodiment 2
[0044] Example 2: Effects on Vascular Permeability of Scalded Rats
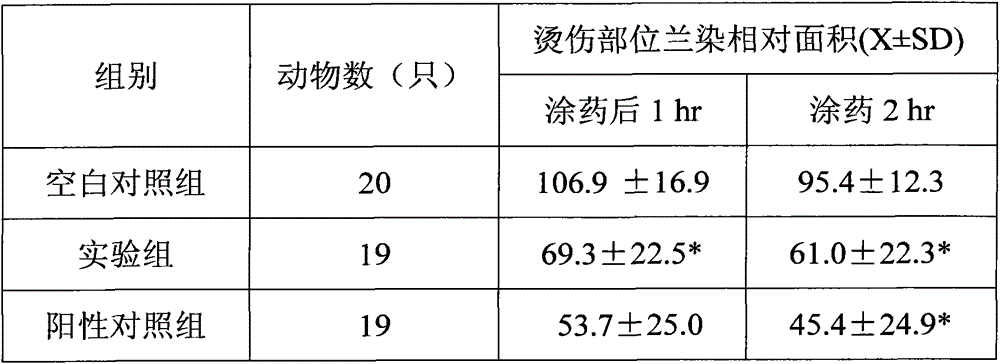
[0045] Take 20 white rats weighing 180-200g, anesthetize them by intraperitoneal injection of 1g / kg Uradum, fix the back, wash with 10% sodium sulfide abdominal hair removal saline and wait until dry, inject 1% Evans blue 0.2mL / rat sublingually Immediately use 80°C water to mark three symmetrical scald surfaces of 1.4×1.4cm on the abdomen, one is the blank control group (no drug application), one is the experimental group (that is, where the biofilm device is used), and one is the positive control group (i.e. use Jingwanhong scald medicine), observe the degree of blue staining on the scalded surface after 1 hr and 2 hr respectively after applying the scald medicine or biofilm, measure and calculate the blue staining area, the results are shown in the table below:
[0046]
[0047] *P<0.01 compared with the control group
[0048] It can be seen that the biofilm device can significantly reduce the vascular ...
Embodiment 3
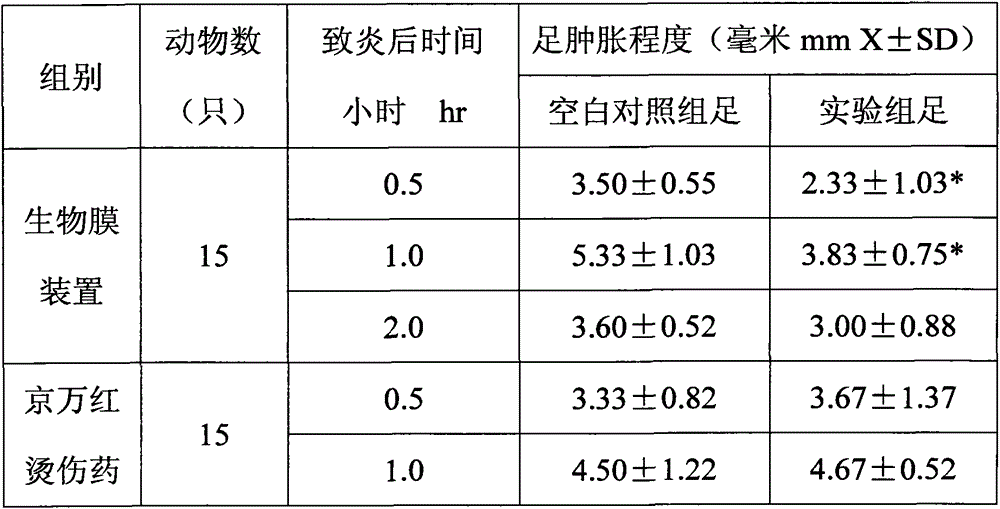
[0049] Example 3: Anti-inflammatory effect
[0050] Thirty white rats with a body weight of 200-220 g were randomly divided into 2 groups, 15 rats per group, and the circumference of the ankle joints and soles of the two hind feet were measured. Subcutaneously inject 20% fresh egg 0.1mL / foot into the ankle joint from the palmar aponeurosis of the foot, and the right hind foot is used as the experimental group. The right hind foot of one group of rats is immediately pasted with the biofilm in the biofilm device, and the right hind foot of the other group of rats is immediately pasted with biofilm in the biofilm device. Immediately apply Jingwanhong scald medicine to the right hind foot, and repeat the film or drug application every 0.5hr. The left hind feet of all the rats in the above two groups are used as the blank control group. At 0.5hr, 1hr, and 2hr after egg white injection, the two hindfoot joints and the meridians of the soles were re-measured, and the degree of inflam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com