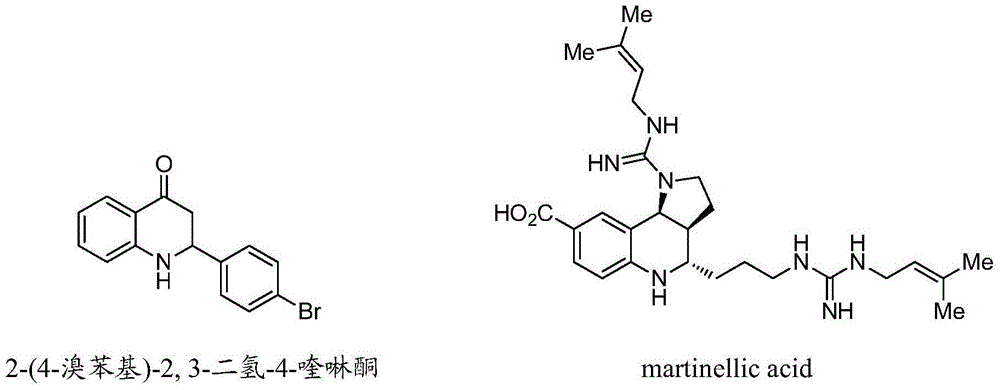
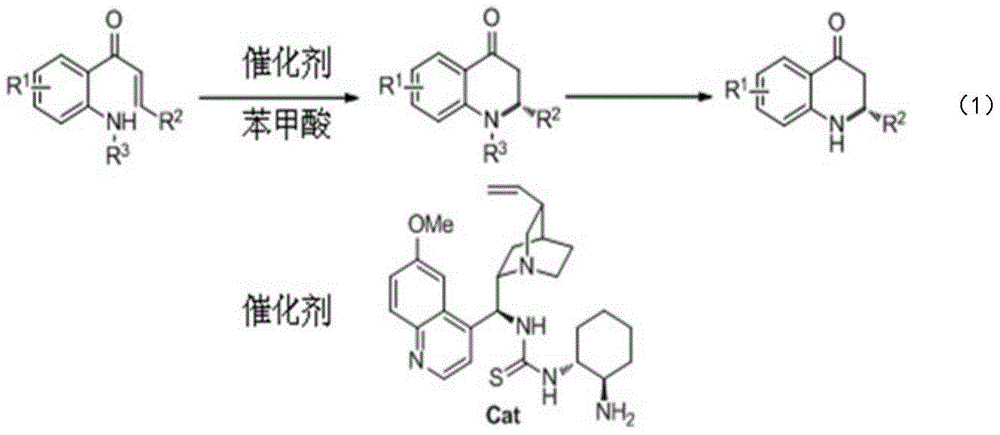
Synthetic method for high-enantioselectivity N-acetyl-2-substitued-2, 3-dihydro-4-quinolinone compounds
An enantioselective, quinolinone-based technology, applied in the direction of heterocyclic compound active ingredients, organic active ingredients, organic chemistry, etc., can solve problems such as unpreparable, unobtainable, inconsistent steps and atom economy requirements, etc. , to achieve the effect of simple preparation and high enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: the synthesis of N-acetyl-2-phenyl-2,3-dihydro-4-quinolinone (Va)
[0022] Add 1-(2-N-acetylaminophenyl)-3-phenyl-2,3-unsaturated acetone 0.1mmol in the reaction flask, quinine-derived thiourea 0.02mmol (catalyst shown in the technical scheme part Structural formula), benzoic acid 0.06mmol and toluene 1mL, stirred at 90°C for 48 hours, purified by column chromatography after reaction to obtain N-acetyl-2-phenyl-2,3-dihydro-4-quinolinone (Va) , the yield was 84%, 1 H NMR (400MHz, CDCl 3 )δ7.94-7.91(m,1H),7.48–7.44(m,1H),7.22–7.13(m,7H),6.47(s,1H),3.37(dd,J=18.0,1.6Hz,1H) ,3.25(dd,J=18.0,5.6Hz,1H),2.43(s,3H). The substance has strong anti-cancer activity (Y.Xia, Z.-Y.Yang, P.Xia, K.F.Bastow, Y.Tachibana, S.-C.Kuo, E.Hamel, T.Hackl, K.-H. Lee, J. Med. Chem. 1998, 41, 1155).
Embodiment 2
[0023] Embodiment 2: Synthesis of N-acetyl-2-(4-methylphenyl)-2,3-dihydro-4-quinolinone (Vb)
[0024] Reaction conditions and purification steps are as N-acetyl-2-phenyl-2,3-dihydro-4-quinolinone (Va) in embodiment 1, by raw material 1-(2-N-acetyl-aminobenzene base)-3-(4-methylphenyl)-2,3-unsaturated acetone (Ⅲb) to synthesize the target product Ⅴb with a yield of 77%. 1 H NMR (400MHz, CDCl 3)δ7.92(d,J=6.8Hz,1H),7.48–7.44(m,1H),7.29(br,1H),7.187.14(m,1H),7.06(d,J=8.0Hz,1H ),6.99(d,J=8.1Hz,1H),6.42(s,1H),3.34(dd,J=18,1.7Hz,1H),3.22(dd,J=18,5.8Hz,1H),2.42 (s,1H),2.21(s,1H).
Embodiment 3
[0025] Embodiment 3: Synthesis of N-acetyl-2-biphenyl-2,3-dihydro-4-quinolinone (Vc)
[0026] Reaction conditions and purification steps are as N-acetyl-2-phenyl-2,3-dihydro-4-quinolinone (Va) in embodiment 1, by raw material 1-(2-N-acetyl-aminobenzene base)-3-biphenyl-2,3-unsaturated acetone (Ⅲc) to synthesize the target product (Ⅴc) with a yield of 77%. 1 H NMR (400MHz, CDCl 3 )δ7.95(dd,J=7.6,1.2Hz,1H),7.50–7.16(m,12H),6.51(s,1H),3.40(dd,J=18.0,1.6Hz,1H),3.27(dd ,J=18.0,6.0Hz,1H),2.45(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com