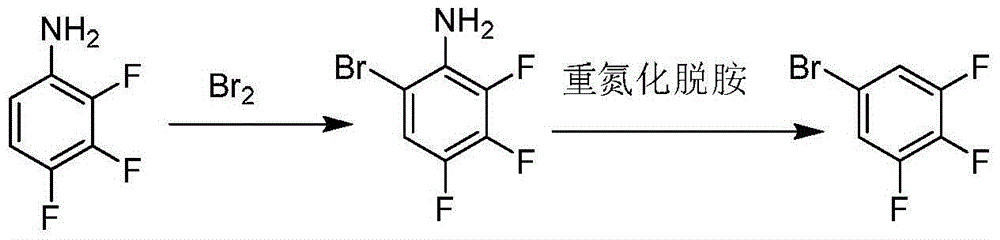
Preparation method of 3, 4, 5-trifluoro bromobenzene
A technology of trifluorobromobenzene and trifluoroaniline is applied in the preparation of pesticide intermediates and in the field of medicine, and can solve the problems of side reactions, many impurities in the product, poor regioselectivity, etc., so as to improve yield, reduce by-products, reduce The effect of production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] In a 500ml three-neck flask, add 14.7g (0.1mol) of 2,3,4-trifluoroaniline, 100ml of water, control the temperature below 10°C, add 19.2g (1.2mol) of bromine dropwise, after the dropwise addition is complete, keep the temperature for 1h. After static separation, an organic oil layer and a water layer were obtained. The organic oil layer was crude 2,3,4-trifluoro-6-bromoaniline, which was used in the next step of reaction; the water layer was collected and recycled.
[0036] Add the crude product of 2,3,4-trifluoro-6-bromoaniline obtained from the above reaction into a 500ml three-necked flask, and add 40ml of 98% concentrated sulfuric acid dropwise under stirring. Add 28ml of sodium nitrite solution with a fraction of 30%. After the dropwise addition, keep it warm for 1 hour and set aside.
[0037] Add 100ml of hypophosphorous acid solution and 0.1g of CuCl into a 500ml three-neck flask, stir, heat up to 40-45°C, add the reacted diazonium solution dropwise, after the dro...
Embodiment 2
[0041] In a 500ml three-necked flask, add 14.7g (0.1mol) of 2,3,4-trifluoroaniline, 100ml of the aqueous phase collected in Example 1, and control the temperature below 10°C, add dropwise 19.2g (1.2mol) of bromine, After the dropwise addition is completed, keep warm for 1 hour, let stand and separate layers to obtain an organic oil layer and a water layer. The organic oil layer is the crude product of 2,3,4-trifluoro-6-bromoaniline, which will enter the next step of reaction; the water layer will be recycled after being collected. .
[0042] Add the crude product of 2,3,4-trifluoro-6-bromoaniline obtained from the above reaction into a 500ml three-necked flask, and add 40ml of 98% concentrated sulfuric acid dropwise under stirring. Add 28ml of sodium nitrite solution with a fraction of 30%. After the dropwise addition, keep it warm for 1 hour and set aside.
[0043] Add 100ml of hypophosphorous acid solution and 0.1g of CuCl into a 500ml three-neck flask, stir, raise the temp...
Embodiment 3
[0046] In a 500ml three-neck flask, add 14.7g (0.1mol) of 2,3,4-trifluoroaniline and 100ml of dichloromethane, and control the temperature below 10°C, add 19.2g (0.12mol) of bromine dropwise, after the dropwise addition is complete, keep the temperature 1h, then add sodium sulfite aqueous solution to neutralize excess bromine and hydrogen bromide, extract twice with dichloromethane, evaporate dichloromethane to dryness to get 2,3,4-trifluoro-6-bromoaniline crude product, set aside .
[0047]Add the crude product of 2,3,4-trifluoro-6-bromoaniline obtained from the above reaction into a 500ml three-necked flask, and add 40ml of 98% concentrated sulfuric acid dropwise under stirring. Add 28ml of sodium nitrite solution with a fraction of 30%. After the dropwise addition, keep it warm for 1 hour and set aside.
[0048] Add 100ml of hypophosphorous acid solution and 0.1g of CuCl into a 500ml three-neck flask, stir, heat up to 40-45°C, add the reacted diazonium solution dropwise, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com