Preparation method of veterinary albendazole/ivermectin dry suspension
A technology of dry albendazol ivermectin and ivermectin, which is applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, block transportation, etc., and can solve poisoning, low drug efficacy, and ivermectin Uneven mixing and other problems, to achieve the effect of convenient use, improved drug efficacy, and eliminate toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
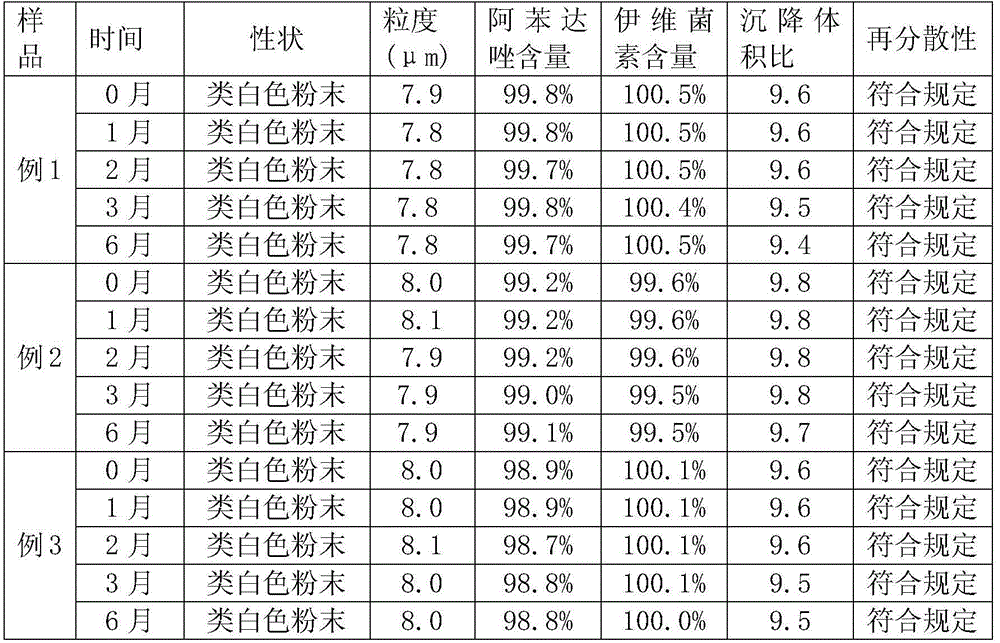
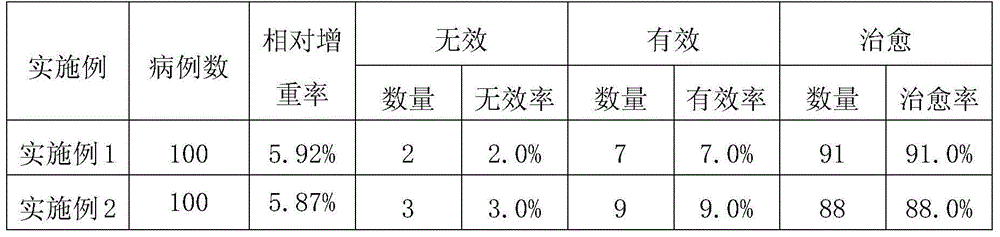
Embodiment 1
[0031] 1. Formula
[0032] Albendazole 10g, ivermectin 0.2g, sodium chloride 10g, sodium citrate 7.5g, sodium carboxymethylcellulose 2g, sodium saccharin 2g, soluble starch 68.3g.
[0033] 2. Preparation method
[0034] The first step: Stir and mix the ivermectin and sodium carboxymethyl cellulose in the prescription amount, add sodium saccharin after the powder is uniform, and mix again to obtain A powder, which is set aside;
[0035] Step 2: Take the prescribed amount of albendazole and sodium chloride and stir and mix. After the powder is uniform, add sodium citrate and mix evenly again to prepare B powder;
[0036] Step 3: Add the above B powder to A powder according to the same addition method and stir evenly, then add enough soluble starch and continue to stir and mix for 10 minutes. The albendazole ivermectin powder of off-white powder is obtained.
Embodiment 2
[0038] 1. Formula
[0039] Albendazole 15g, ivermectin 0.2g, sodium chloride 20g, sodium citrate 10g, sodium carboxymethylcellulose 2g, sucrose 2g, soluble starch 50.8g.
[0040] 2: Preparation method: the same as the method described in Example 1.
Embodiment 3
[0042] 1. Formula
[0043] Albendazole 5g, ivermectin 0.1g, sodium chloride 10g, sodium citrate 5g, sodium carboxymethylcellulose 4g, sucrose 2g, soluble starch 73.9g.
[0044] 2: Preparation method: the same as the method described in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com