Freeze-dried powder of pravastatin sodium composition for injection
A technology of pravastatin sodium and freeze-dried powder injection, which is applied in the field of medicine and medicine manufacturing, can solve the problem of no chitosan-containing nanoparticles, etc., and achieve the effects of reducing adverse reactions, eliminating active effects and enhancing lipid-lowering effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
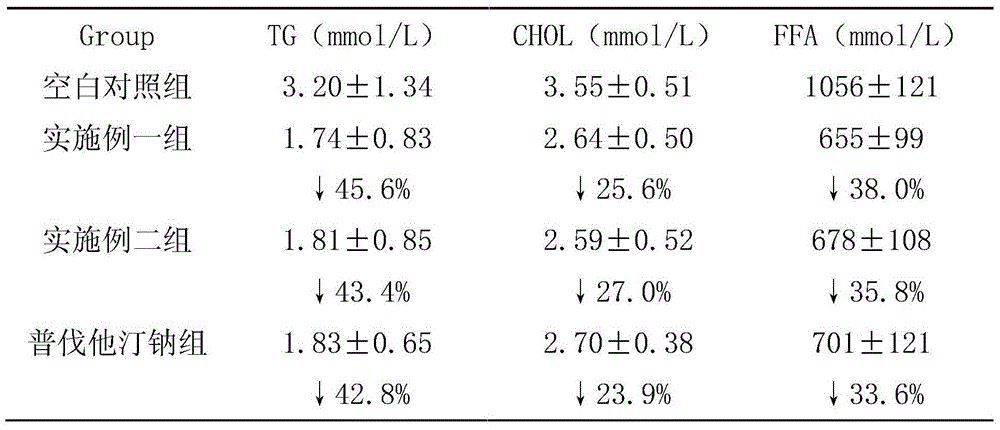
Examples
Embodiment 1
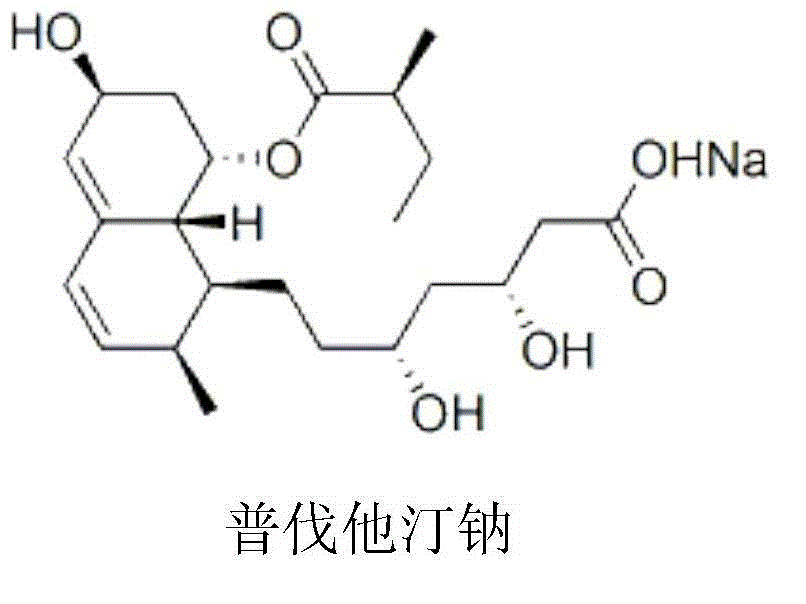
[0039] Example 1. Preparation of pravastatin sodium composition freeze-dried powder for injection (specification: 10 mg, calculated as pravastatin sodium), in 1000 vials.
[0040] 1. Prescription:
[0041] Pravastatin Sodium 10g
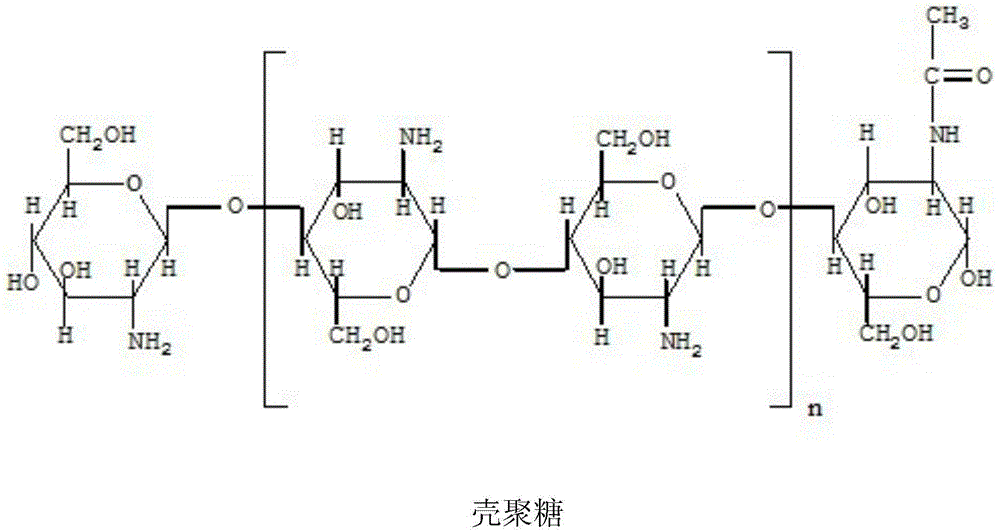
[0042] Chitosan Nanoparticles 8g
[0043] Water for injection 2000ml
[0044] 2. Preparation process:
[0045] 1) Slowly add 8g of chitosan nanoparticles into the prescribed amount of water for injection (theoretical loading 2ml / bottle), and stir until dissolved while adding.
[0046] 2) Continue to add 10g of pravastatin sodium and stir to dissolve until clear.
[0047] 3) Use sodium hydroxide to adjust the pH to 5.5-6.0, add activated carbon with 0.1% volume of water for injection and stir for 30 minutes; then decarburize and circulate and filter through titanium rods for 30 minutes; then sterilize through 0.45 μm and 0.22 μm Circulate filtration for 30 minutes; detect the content of intermediates, and calculate the filling amount based on 10 ...
Embodiment 2
[0049] Embodiment 2, preparation of pravastatin sodium composition freeze-dried powder for injection (specification: 10 mg, calculated as pravastatin sodium), calculated in 1000 pieces.
[0050] 1. Prescription:
[0051] Pravastatin Sodium 10g
[0052] Chitosan Nanoparticles 9g
[0053] Water for injection 2000ml
[0054] 2. Preparation process:
[0055] 1) Slowly add 9g of chitosan nanoparticles into the prescribed amount of water for injection (theoretical loading 2ml / bottle), and stir until dissolved while adding.
[0056] 2) Continue to add 10g of pravastatin sodium and stir to dissolve until clear.
[0057] 3) Use sodium hydroxide to adjust the pH to 5.5-6.0, add activated carbon with 0.1% volume of water for injection and stir for 30 minutes; then decarburize and circulate and filter through titanium rods for 30 minutes; then sterilize through 0.45 μm and 0.22 μm Circulate filtration for 30 minutes; detect the content of intermediates, and calculate the filling amount...
Embodiment 3
[0059] Example 3. Preparation of pravastatin sodium composition freeze-dried powder for injection (specification: 10 mg, calculated as pravastatin sodium), in 1000 vials.
[0060] 1. Prescription:
[0061] Pravastatin Sodium 10g
[0062] Chitosan Nanoparticles 7g
[0063] Water for injection 2000ml
[0064] 2. Preparation process:
[0065] 1) Slowly add 7g of chitosan nanoparticles into the prescribed amount of water for injection (theoretical loading 2ml / bottle), and stir until dissolved while adding.
[0066] 2) Continue to add 10g of pravastatin sodium and stir to dissolve until clear.
[0067] 3) Use sodium hydroxide to adjust the pH to 5.5-6.0, add activated carbon with 0.1% volume of water for injection and stir for 30 minutes; then decarburize and circulate and filter through titanium rods for 30 minutes; then sterilize through 0.45 μm and 0.22 μm Circulate filtration for 30 minutes; detect the content of intermediates, and calculate the filling amount based on 10 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com