Preparation method for ivermectin sustained-release gelatin microcapsule
A technology of ivermectin and sustained-release gelatin, which is applied in the field of preparation of sustained-release gelatin microcapsules, can solve the differences in product particle size, drug-loading biocompatibility and drug-releasing characteristics, and restrict the selection of formulations and longevity. It can achieve the effect of low price, simple production process and equipment requirements, and improved encapsulation rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
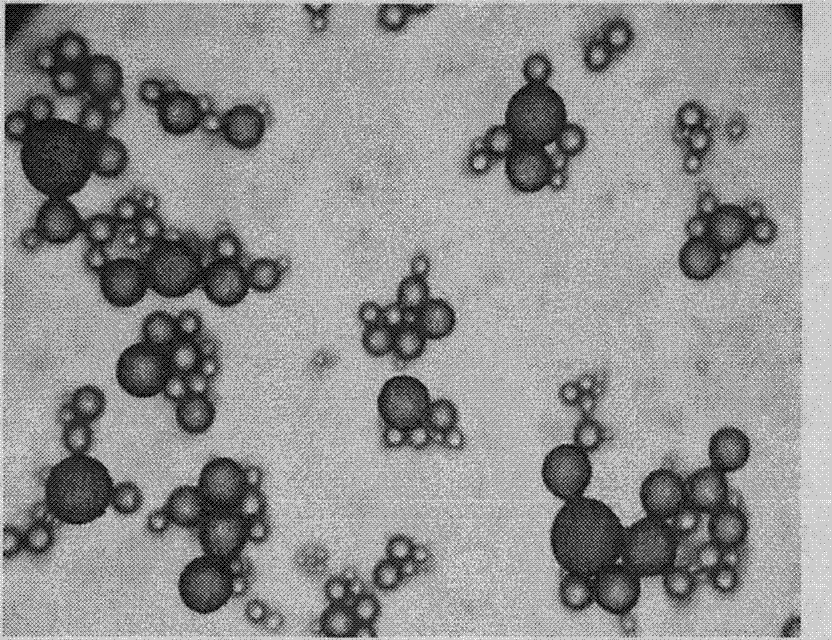
Image
Examples
Embodiment 1
[0014] The specific preparation steps of ivermectin sustained-release gelatin microcapsules are:
[0015] 1. Preparation of raw and auxiliary materials: Micronize the fine ivermectin raw powder crystalline powder visible to the naked eye, so that the particle size of the powder is less than 3um to prepare ivermectin micropowder; take an appropriate amount of 10% type B gelatin solution, press V / W200:1 ratio is added, fully dissolved to obtain compound gelatin solution; 30% sodium sulfate solution; 15% sodium sulfate solution; glycerin; formaldehyde solution;
[0016] 2. Preparation method:
[0017] (1) Take an appropriate amount of ivermectin (IVM) micropowder, and then quickly and fully emulsify it in glycerin at a ratio of 1:2 (W / V) to form a uniform yellow-white paste;
[0018] (2) According to the ratio of IVM micropowder: compound gelatin solution of 1.5:40 (W / V), take an appropriate amount of compound gelatin solution corresponding to the amount of IVM, and then add the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com