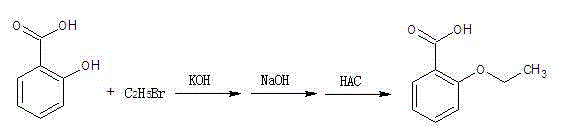
Preparation method of o-ethoxybenzoic acid
A technology of o-ethoxybenzoic acid and salicylic acid, which is applied in the field of preparation of o-ethoxybenzoic acid, can solve problems such as difficulty in realizing large-scale industrial production, uneasy control of reaction conditions, unfavorable industrial production, etc., and facilitates industrialization The effect of low production, low cost and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Add 50g (0.36mol) of salicylic acid and 500ml of acetone into the reaction flask, and add 42.6g (0.76mol) of finely ground potassium hydroxide solid (90%) at a temperature of less than 40°C. After the addition, raise the temperature and keep it under reflux for 3 hours , cooled to 20°C, slowly added 86g (0.79mol) ethyl bromide dropwise, after the addition of ethyl bromide was completed, the temperature was raised to reflux, and the reflux state was maintained for 10 hours. After the heat preservation, the acetone was recovered under reduced pressure. After the acetone recovery was completed, 300ml was added Water, 50 grams of 30% liquid caustic soda (that is, liquid sodium hydroxide) (0.38mol) is heated up to 90°C, and kept at this temperature for 2 hours, and cooled to 30-35°C after the heat preservation, and slowly dripped Add glacial acetic acid, control the reaction liquid to be added dropwise to PH=3~4, at this time a large amount of solids precipitated, further coo...
Embodiment 2
[0014] Add 65g (0.47mol) of salicylic acid and 400ml of acetone into the reaction flask, and add 67.3g (1.08mol) of finely ground potassium hydroxide solid (90%) at a temperature of less than 40°C. After the addition, raise the temperature and keep it under reflux for 3 hours , cooled to 20°C, and slowly added 117.8g (1.08mol) bromoethane dropwise. After the dropwise addition of bromoethane, the temperature was raised to reflux, and the reflux state was maintained for 12 hours. After the heat preservation was completed, acetone was recovered under reduced pressure. After the acetone recovery was completed, add 500ml of water, 62.7 grams of 30% liquid caustic soda (that is, liquid sodium hydroxide) (0.47mol) is heated up to 90°C, and kept at this temperature for 3 hours, and cooled to 30-35°C at the end of the heat preservation, and slowly Add glacial acetic acid dropwise, and control the dropwise addition of the reaction solution to PH=3~4. At this time, a large amount of solid...
Embodiment 3
[0016] Add 100g (0.72mol) of salicylic acid and 900ml of acetone into the reaction flask, and add 112.2g (1.80mol) of finely ground potassium hydroxide solid (90%) at a temperature control of less than 40°C. After adding, raise the temperature and keep it under reflux for 3 hours , cooled to 20°C, slowly added 196g (1.80mol) ethyl bromide dropwise, after the addition of ethyl bromide was completed, the temperature was raised to reflux, and the reflux state was maintained for 15 hours. After the heat preservation, the acetone was recovered under reduced pressure. After the acetone recovery was completed, 600ml was added Water, 96 grams of 30% liquid caustic soda (that is, liquid sodium hydroxide) (0.72mol) is heated up to 90°C, and kept at this temperature for 4 hours, and cooled to 30-35°C after the heat preservation, and slowly dripped Add glacial acetic acid, control the reaction liquid to be added dropwise to PH = 3 ~ 4, at this time, a large amount of solids precipitated, f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com