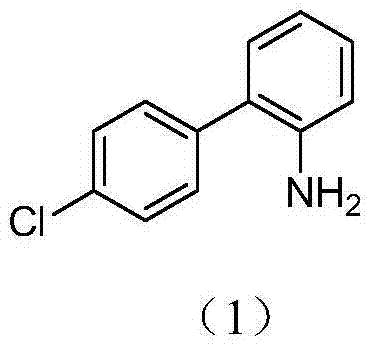
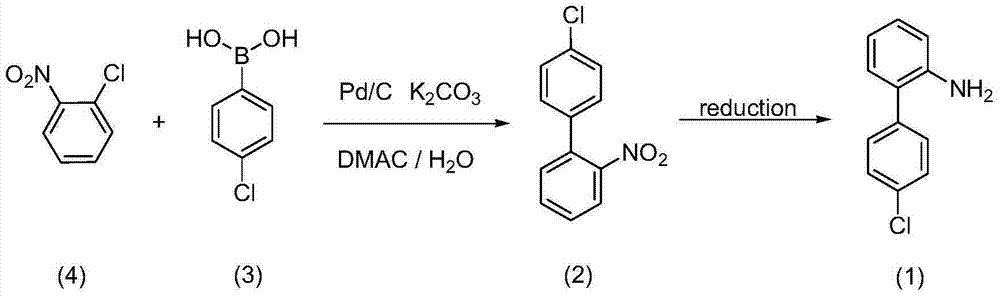
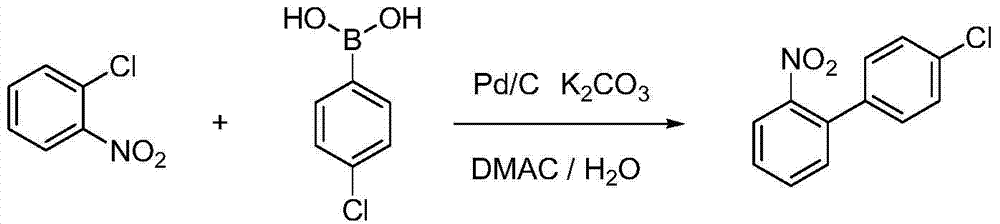
Method for preparing 4'-chloro-2-aminobiphenyl through palladium/carbon catalysis
A technology for catalytic preparation and aminobiphenyl, which is applied in the preparation of amino compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of high price, difficult to recycle and reuse, and increased reaction cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 0.16g o-chloronitrobenzene, 0.23g p-chlorophenylboronic acid are added in the there-necked flask, add 0.41g potassium phosphate, 0.036g palladium carbon (5% metal content, 69.13% water content), 15mL tetrahydrofuran (molar ratio: o Chloronitrobenzene: p-chlorophenylboronic acid: palladium on carbon: potassium phosphate: tetrahydrofuran = 1:1.5:0.017:1.9:166), heated to 70°C for 12 hours. Stop the reaction, cool down to room temperature, filter with suction, recover the filter cake, evaporate the filtrate to remove the solvent under reduced pressure, add dichloromethane and water to extract the residue, evaporate the solvent to dryness in the organic layer, precipitate a solid, wash with petroleum ether twice, and filter with suction to obtain 4'-Chloro-2-nitrobiphenyl, yield 24.32%. The reaction structure is as follows:
[0033]
Embodiment 2
[0035] 0.16g o-chloronitrobenzene, 0.23g p-chlorophenylboronic acid joins in the there-necked flask, add 0.41g potassium carbonate, 0.036g palladium charcoal, 15mL toluene (the molar ratio is: o-chloronitrobenzene: p-chlorophenylboronic acid: palladium Carbon: Potassium Carbonate: Toluene=1:1.5:0.017:3:130), the temperature was raised to 100°C for 12h. Stop the reaction, cool down to room temperature, filter with suction, recover the filter cake, evaporate the filtrate to remove the solvent under reduced pressure, add dichloromethane and water to extract the residue, evaporate the solvent to dryness in the organic layer, precipitate a solid, wash with petroleum ether twice, and filter with suction to obtain 4'-Chloro-2-nitrobiphenyl, yield 34.51%.
Embodiment 3
[0037] 0.16g o-chloronitrobenzene, 0.23g p-chlorophenylboronic acid joins in the there-necked flask, adds 0.41g potassium carbonate, 0.020g palladium carbon (molar ratio is: o-chloronitrobenzene: p-chlorophenylboronic acid: palladium carbon: carbonic acid Potassium=1:1.5:0.009:3), 15g of DMAC and 1g of water (weight ratio: water:DMAC=1:15) were heated to 130°C for 12h. Stop the reaction, cool down to room temperature, filter with suction, recover the filter cake, evaporate the filtrate to remove the solvent under reduced pressure, add dichloromethane and water to extract the residue, evaporate the solvent to dryness in the organic layer, precipitate a solid, wash with petroleum ether twice, and filter with suction to obtain 4'-Chloro-2-nitrobiphenyl, yield 34.16%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com