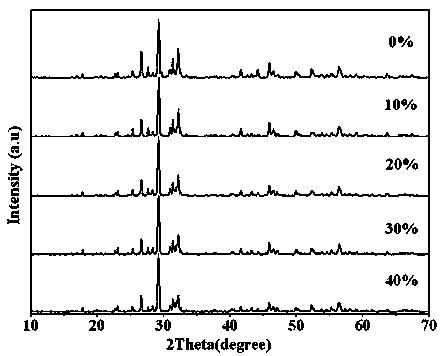
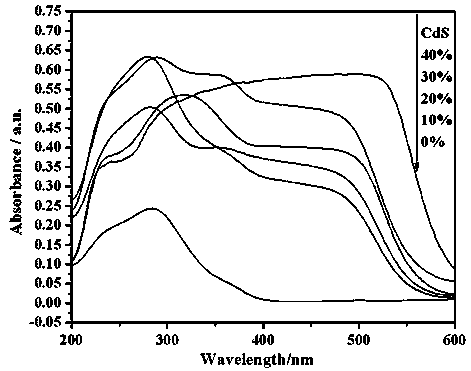
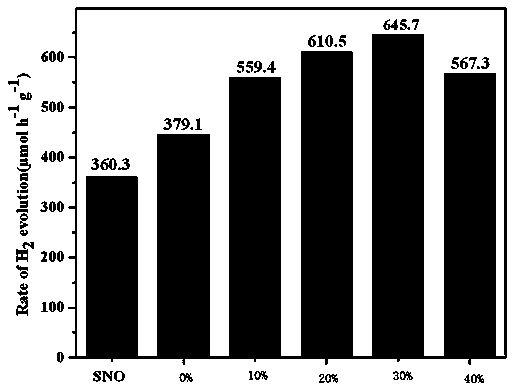
Photocatalytic water-splitting hydrogen production material CdS/Sr1.6Zn0.4Nb2O7 and preparation method thereof
A sr1.6zn0.4nb2o7, water splitting technology, applied in chemical instruments and methods, chemical/physical processes, physical/chemical process catalysts, etc., can solve the problem of easy recombination of photogenerated electrons and holes, low utilization rate of visible light, and preparation conditions Harsh and other problems, to achieve the effect of easy reuse, low production cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Weigh 1.5949 g (6 mmol) Nb 2 o 5 In a plastic beaker, add 10 ml of HF and place in a water bath at 80°C to dissolve until clear, add ammonia water dropwise to adjust the pH to greater than 9, age at 80°C for 2 hours, and purify with deionized water 3 to 4 times during this period. Suction filtration, washing and drying. Dissolve the dried sample in 7.5650 g (36 mmol) of citric acid solution, add 3 ml of hydrogen peroxide, and add 2.0316 g (96 mmol) of strontium nitrate and 0.7140 g (24 mmol) of zinc nitrate after the solution is clarified and 5.0434 g (24 mmol) of citric acid, stirred and dissolved in sol at 80°C, dried at 100°C, calcined at 900°C for 10 h, and ground to obtain Sr 1.6 Zn 0.4 Nb 2 o 7 powder.
Embodiment 2
[0020] Catalyst 10%CdS / Sr 1.6 Zn 0.4 Nb 2 o7 Synthesis. Weigh 2 g of the prepared Sr 1.6 Zn 0.4 Nb 2 o 7 Disperse the powder in 30 ml of water, stir evenly, add dropwise a solution made of 0.1149 g of cadmium acetate, ultrasonically disperse for 15 minutes, add dropwise a solution made of 0.0328 g of thiourea, ultrasonically disperse for 15 minutes, and put the resulting solution into a 100 ml container In a polytetrafluoroethylene reactor, put it into a muffle furnace at 150°C for hydrothermal reaction for 10 hours. The sample after the hydrothermal reaction is filtered, washed, dried and ground for many times to obtain the target catalyst.
Embodiment 3
[0022] Catalyst 20%CdS / Sr 1.6 Zn 0.4 Nb 2 o 7 Synthesis. Weigh 2 g of the prepared Sr 1.6 Zn 0.4 Nb 2 o 7 Disperse the powder in 30 ml of water, stir evenly, add dropwise a solution made of 0.2297 g of cadmium acetate, ultrasonically disperse for 15 minutes, add dropwise a solution made of 0.0656 g of thiourea, ultrasonically disperse for 15 minutes, and put the resulting solution into a 100 ml container In a polytetrafluoroethylene reactor, put it into a muffle furnace at 150°C for hydrothermal reaction for 10 hours. The sample after the hydrothermal reaction is filtered, washed, dried and ground for many times to obtain the target catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com