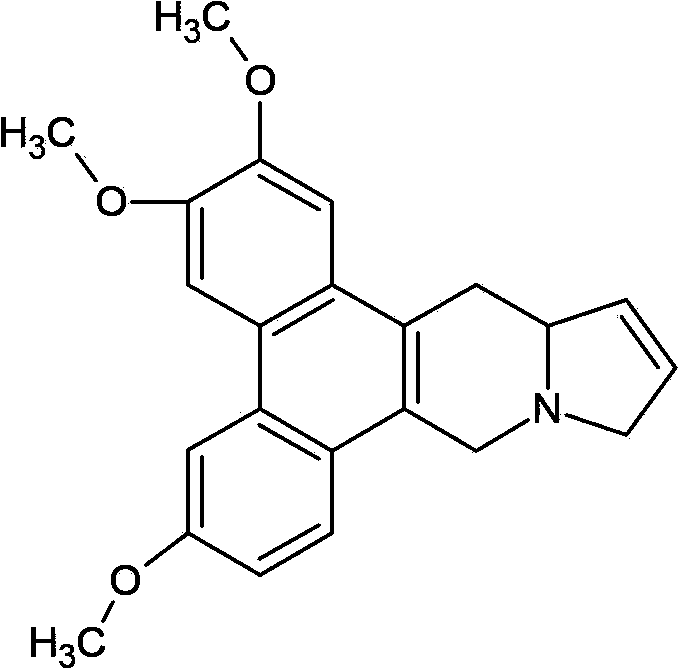
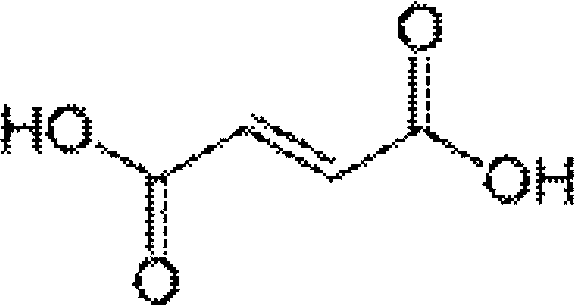
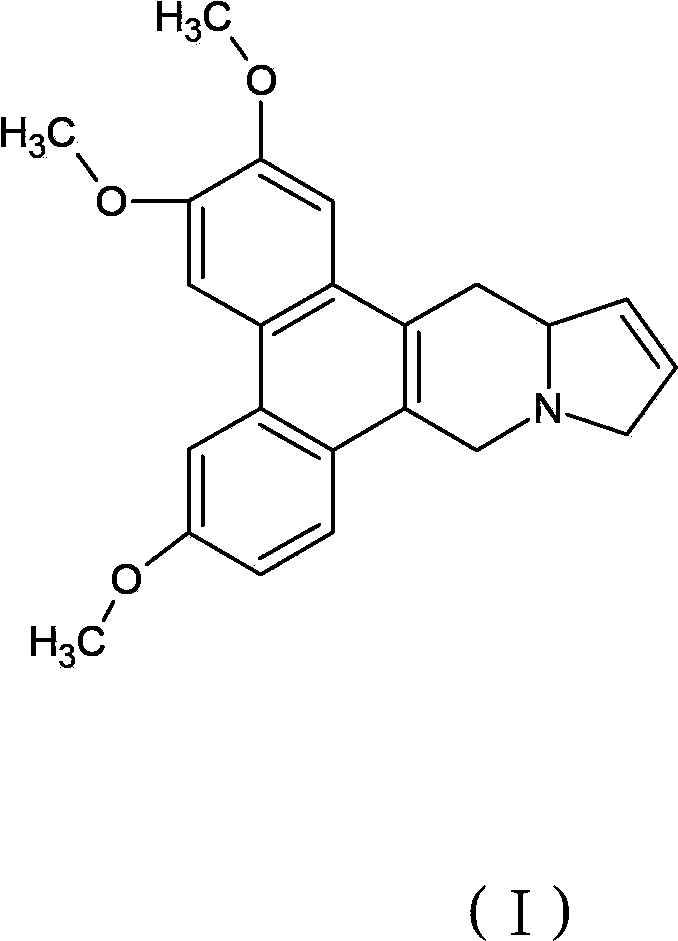
Salts of delta 11,12-antofine derivatives
A technology of tofin and drugs, applied in the field of medicinal chemistry, can solve problems such as adverse reactions, drug resistance, and unsatisfactory therapeutic effects, and achieve the effect of practical therapeutic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Apoprofen fumarate
[0048] Dissolve 3.61 g (0.01 mol) of the compound apoprofen and 1.17 g (0.01 mol) of fumaric acid in 100 ml of boiling ethanol. The hot solution is filtered through diatomaceous earth, then slowly cooled under gentle stirring, and left to stand for several hours at a temperature of 0-5°C to precipitate apoprofen fumarate crystals, and apoprofen fumarate is filtered out Salt crystals were washed with ethanol and dried under vacuum at 50°C to obtain 4.76 g of product.
Embodiment 2
[0050] Apoprofen fumarate
[0051] Stir 3.61 g of the compound apoprofen fumarate and 1.17 g of fumaric acid in 100 ml of refluxing ethanol until all the solids are dissolved. Add activated carbon, filter the hot solution through diatomaceous earth, and cool down to room temperature while stirring. After standing for several hours in a temperature environment of 0-5°C, the crystals of apoantifen fumarate are precipitated, and the crystals of apoantifen fumarate are filtered out, washed with ethanol and dried under vacuum at 50°C to obtain 4.74 g of product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com