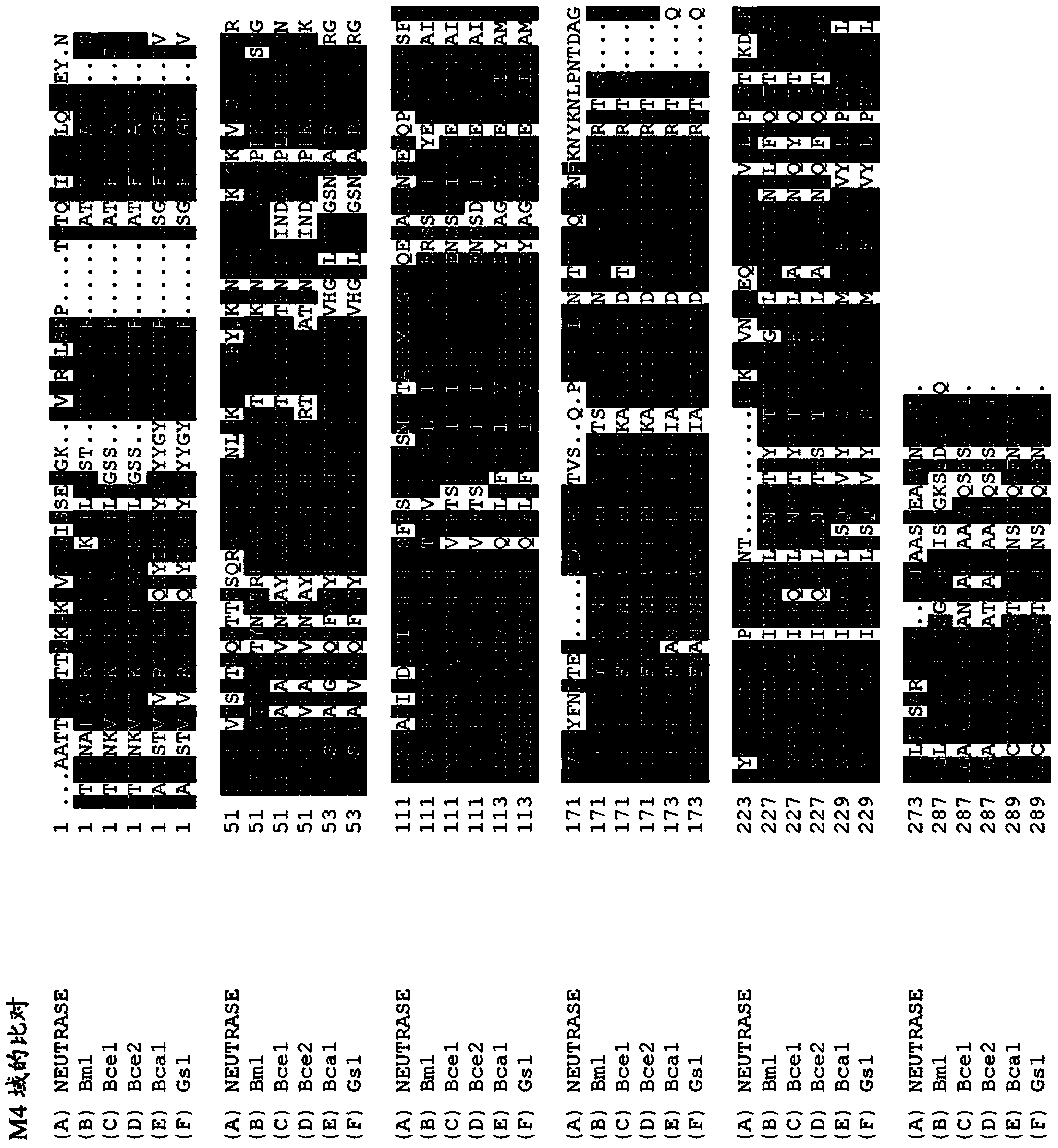
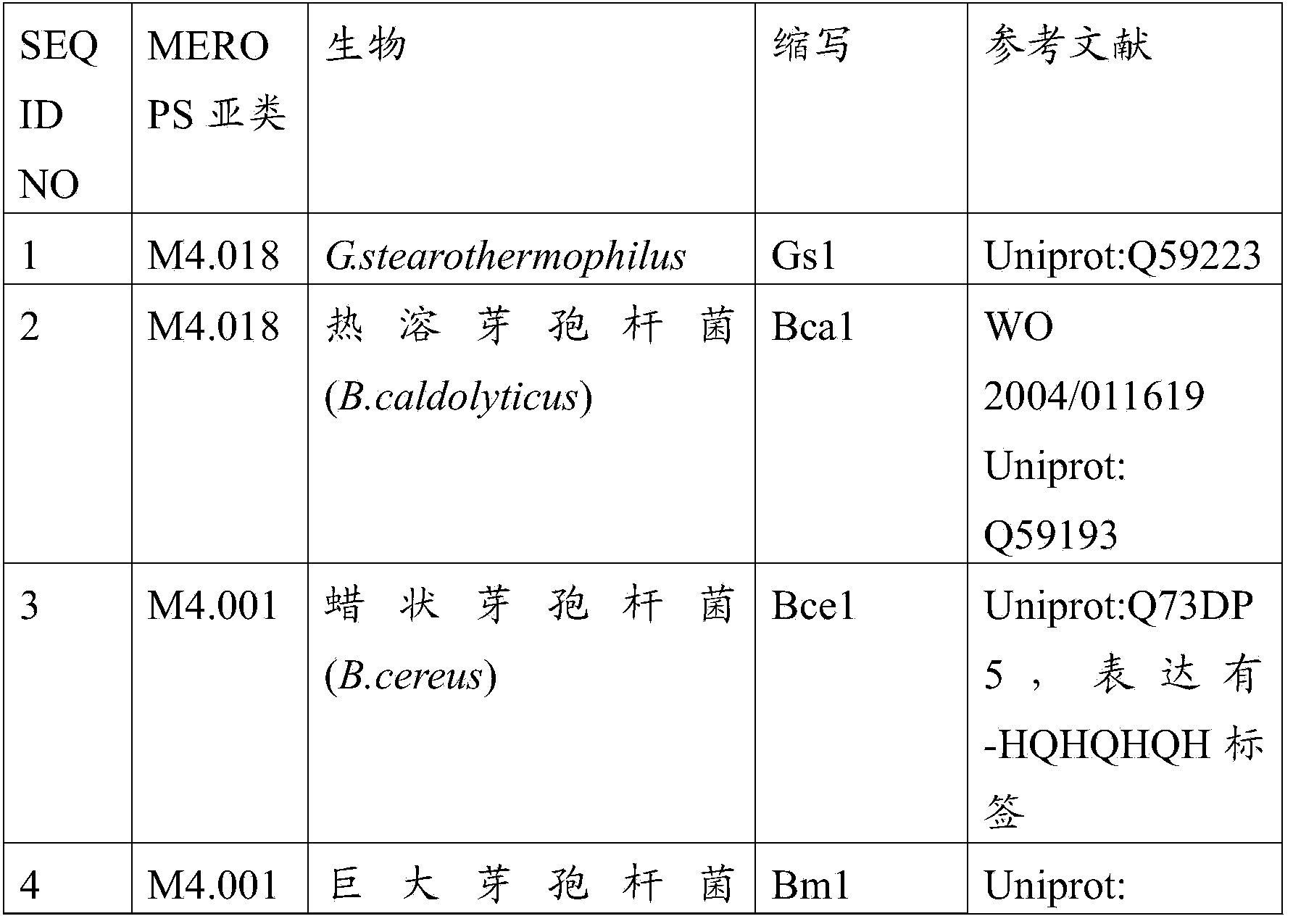
Detergent compositions comprising metalloproteases
A technology of metalloprotease and composition, which is applied in the direction of detergent composition, detergent compounding agent, enzyme, etc., and can solve problems such as restrictions on the use of metalloprotease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0260] Gs1 from Geobacillus stearothermophilus and Bca1 from Bacillus thermolyticus purification of
[0261] Expression of Gs1 and Bca1 proteases in Bacillus subtilis.
[0262] The culture broth was centrifuged (20000 xg, 20 minutes) and the supernatant was carefully decanted from the pellet. The supernatant was filtered through a Nalgene 0.2 μm filter unit to remove remaining Bacillus host cells. The 0.2 μm filtrate was transferred to 50 mM H on a G25sephadex column (from GE Healthcare) 3 BO 3 , 5 mM dimethylglutaric acid, 1 mM CaCl 2 , pH7. G25sephadex-transferred enzyme was applied in 50mM H 3 BO 3 , 5 mM dimethylglutaric acid, 1 mM CaCl 2 , Bacitracin agarose column (from Upfront chromatography) equilibrated in pH 7. After washing the column thoroughly with equilibration buffer, thermolysin was washed with 25% (v / v) 2-propanol in 100mM H 3 BO 3 , 10mM MES, 2mM CaCl 2 , 1M NaCl, pH6 elution. Fractions from the column were analyzed for protease activity (Prot...
Embodiment 2
[0264] Purification of Bm1 from Bacillus megaterium and Bce1 and Bce2 from Bacillus cereus
[0265] Expression of Bm1, Bce1 and Bce2 proteases in Bacillus subtilis.
[0266] The culture broth was centrifuged (20000 xg, 20 minutes) and the supernatant was carefully decanted from the pellet. The supernatant was filtered through a Nalgene 0.2 μm filter unit to remove remaining Bacillus host cells. The 0.2 μm filtrate was transferred to 50 mM H on a G25 sephadex column (from GE Healthcare) 3 BO 3 , 5 mM dimethylglutaric acid, 1 mM CaCl 2 , pH7. G25 sephadex-transferred enzyme was applied in 50mM H 3 BO 3 , 5 mM dimethylglutaric acid, 1 mM CaCl 2 , Bacitracin agarose column (from Upfront chromatography) equilibrated in pH 7. After washing the column thoroughly with equilibration buffer, the M4 protease was washed with 25% (v / v) 2-propanol in 100mM H 3 BO 3 , 10mM MES, 2mM CaCl 2 , 1M NaCl, pH6 elution. Fractions from the column were analyzed for protease activity (Pro...
Embodiment 3
[0268] Characterization of Bm1, Bce1 and Bce2 proteases: pH activity and pH stability
[0269] The Protazyme OL characterization assay was used as described in the "Materials and Methods" section to obtain a pH activity profile at 37°C, as well as a pH stability profile at pH optima (residual activity after 2 hours at the indicated pH values). For the pH stability profile, proteases were diluted 8-fold in different characterization assay buffers to achieve the pH of these buffers and incubated at 37°C for 2 hours. After incubation, the pH of the protease incubation was shifted to the pH optimum of the protease by dilution in pH optimum assay buffer and residual activity was determined. The results are shown in the table below. For Table 3, the activities are relative values to the optimum pH of the samples. For Table 4, the activities are relative values relative to samples maintained at stable conditions (5°C, optimum pH).
[0270] Characterization of Gs1 and Bca1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com