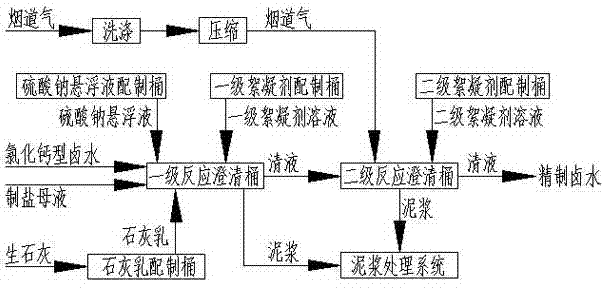
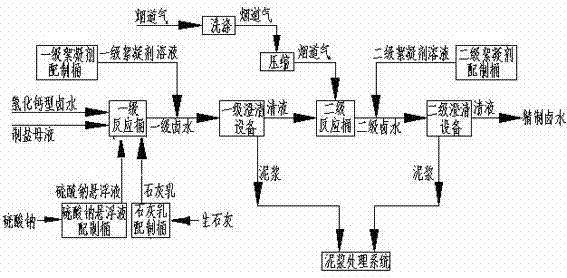
Lime-sodium sulfate-carbon dioxide method used for purifying calcium chloride type bittern
A calcium chloride type brine and carbon dioxide technology, applied in the direction of alkali metal chlorides, etc., can solve the problems of increasing the cost of brine purification, wasting resources, and short brushing cycles, and achieves reduction of purification costs and steam consumption, and carbon dioxide emissions. The effect of quantity and long production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The composition of calcium chloride type brine is: H 2 O: 885.7g / l; NaCl: 310 g / l; CaCl 2 : 2.56 g / l; CaSO 4 : 1.61g / l; MgCl 2 : 0.23 g / l; if the salt workshop produces 10t of industrial salt, the purification workshop needs to process 32.8m 3 Calcium chloride type brine and 27.5m 3 Mother liquor for salt production, primary reaction, first add 147.2kg of Na at 20-100r / min stirring speed 2 SO 4 , then add 56.5kg CaO and continue to stir the reaction for 5 hours, then add 0.12kg of primary flocculant, stop stirring and clarify for 2 hours, measure the Mg in the clear liquid 2+ and PH values are 0.1ppm and 12.5 respectively; transfer the primary clear liquid to the secondary reaction, and the secondary reaction is passed into the flue gas 298Nm at a stirring speed of 20-100r / min 3, continue to stir, add 0.12kg of secondary flocculant after 2 hours, stop stirring and clarify for 2 hours, measure the Ca in the clear liquid 2+ , Mg 2+ and pH value are 4ppm, 0.1ppm ...
Embodiment 2
[0035] The composition of calcium chloride type brine is: H 2 O: 885.2g / l; NaCl: 300 g / l; CaCl 2 : 10.51 g / l; CaSO 4 : 1.91g / l; MgCl 2 : 2.38 g / l; if the salt workshop produces 10t of industrial salt, the purification workshop needs to process 33.7m 3 Calcium chloride type brine and 25.6m 3 Mother liquor for salt production, primary reaction Add 598.9kg of Na at a stirring speed of 20-100r / min 2 SO 4 , then add 99.5kg CaO and ensure that the residence time is more than 5 hours, transfer to the primary clarification equipment, and add 0.12kg of primary flocculant at the same time, measure the Mg in the clear liquid 2+ and PH values are 0.1ppm and 12.5 respectively; the clear liquid of the primary clarification equipment is transferred to the secondary reaction barrel, and the secondary reaction is fed with carbon dioxide 302Nm at a continuous stirring speed of 20-100r / min 3 , and ensure that the residence time is more than 2 hours, transfer to the secondary clarificatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com