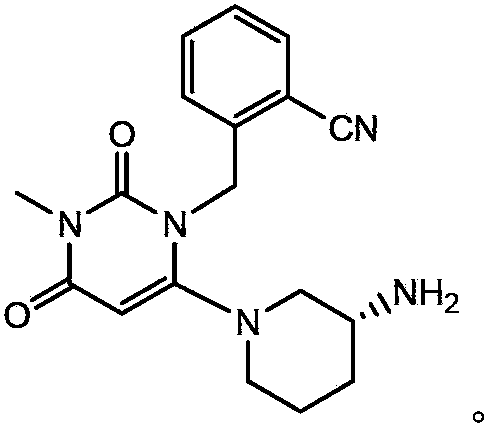
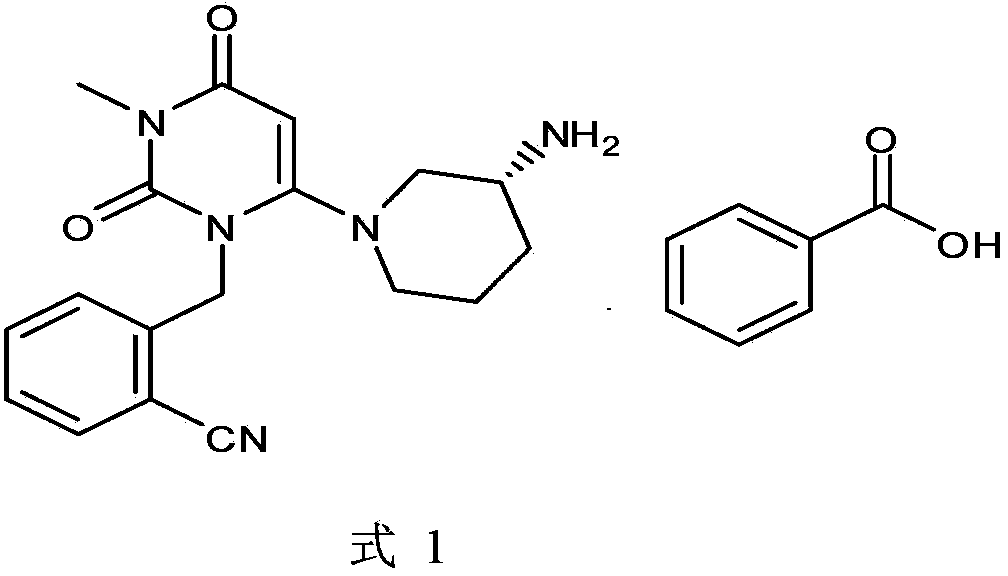
Preparation method of alogliptin benzoate
A technology of benzoic acid and acid hydrolysis, applied in carboxylate preparation, organic chemistry, etc., can solve the problems of low purity and low yield of compound 4, and achieve the effects of simple operation, high reaction yield, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
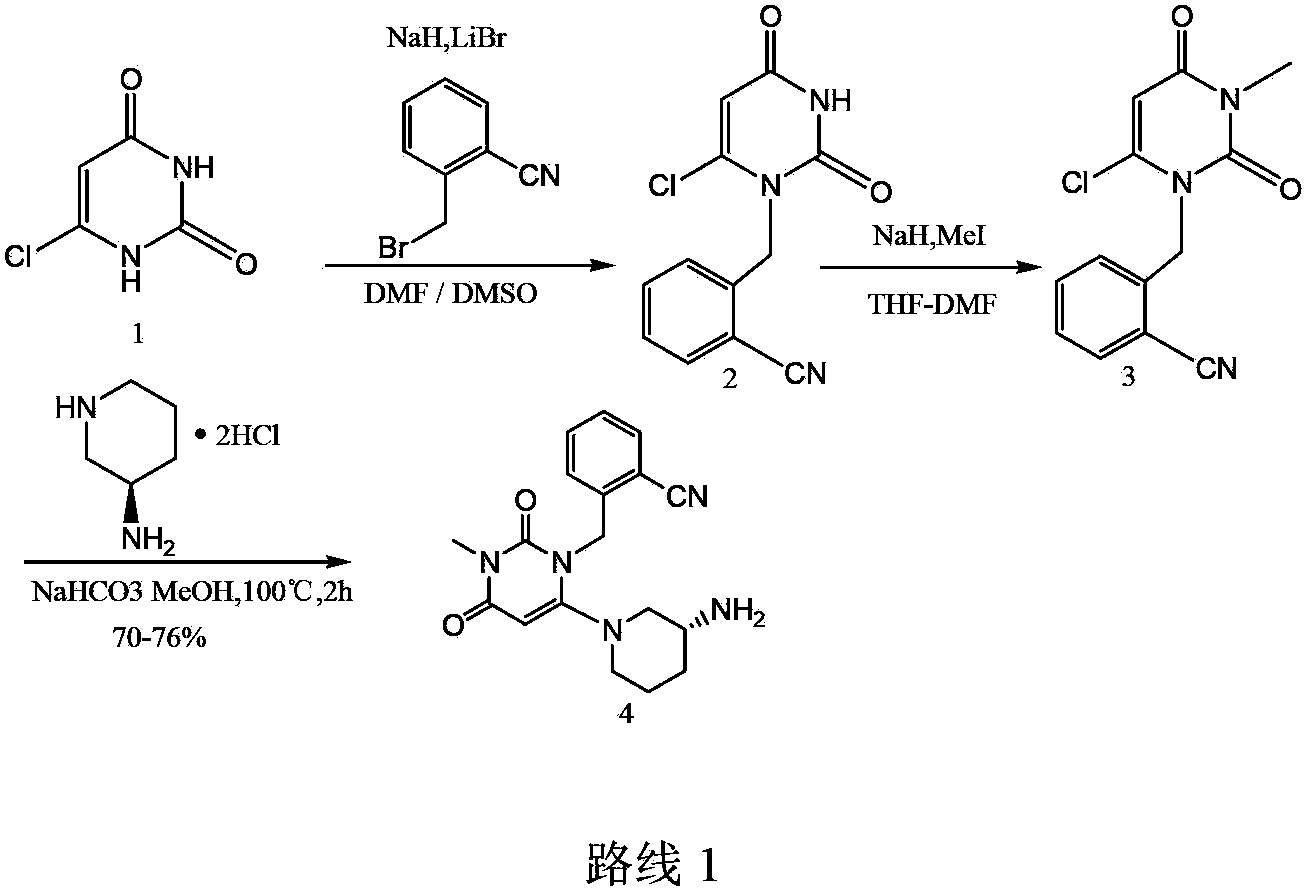
Embodiment 1
[0031] Preparation of Formula 5:
[0032]
[0033] Dissolve (R)-3-amino-piperidine (43g) in 300mL of toluene, add benzaldehyde (45g) dropwise to the above solution at room temperature, continue to stir for 3 hours, distill toluene under reduced pressure to obtain 78g of oil, directly for the next reaction.
[0034] Preparation of Formula 4:
[0035]
[0036] at 0°C, N 2 Under protective conditions, add 6-methyl-3-chlorouracil (200g) into a 10L three-necked flask, dissolve it with DMF / DMSO (4.5L, 5 / 1) solution, add 60% NaH (54.8 g, 1.36mol), stirred for 30 minutes, then added LiBr (87g, 1mol), after continuing to stir for 20 minutes, to which o-cyanobenzyl bromide (244g, 1.25mol) was added dropwise, after reacting for 1 hour, moved to room temperature to continue Reaction, TLC tracked until the raw material reacted completely, and the solvent was evaporated under reduced pressure, and the residue was added with 1.2L water, and the 2 Cl 2 Extraction (1L × 3 times), co...
Embodiment 2
[0053] Preparation of Formula 5:
[0054]
[0055] Dissolve (R)-3-amino-piperidine (50g) in 300mL of toluene, add p-tolualdehyde (66g) dropwise to the above solution at room temperature, continue to stir for 3 hours, evaporate toluene under reduced pressure, and obtain 81g of oil were used directly in the next reaction.
[0056] Preparation of formula 6 and formula 7:
[0057]
[0058] 2-(6-chloro-3-methyl-2,4-dioxo-3,4-dihydro-2-H-pyrimidin-1-ylmethyl)-benzylcyanide (100g), compound of formula 5 ( 80.7g) and sodium bicarbonate (193g) and 34g activated molecular sieve (4A) in anhydrous MeOH (2L), reflux at 100°C, stir for 3h, spot the plate until the reaction is complete, cool to room temperature (25°C), add 1L HCl (3mol / L) and stir for 2 hours until the compound of formula 6 disappears, evaporate the methanol under reduced pressure, wash the water phase with 300ml of dichloromethane, remove the organic layer, adjust the pH of the water phase to 9 with saturated sodium...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com