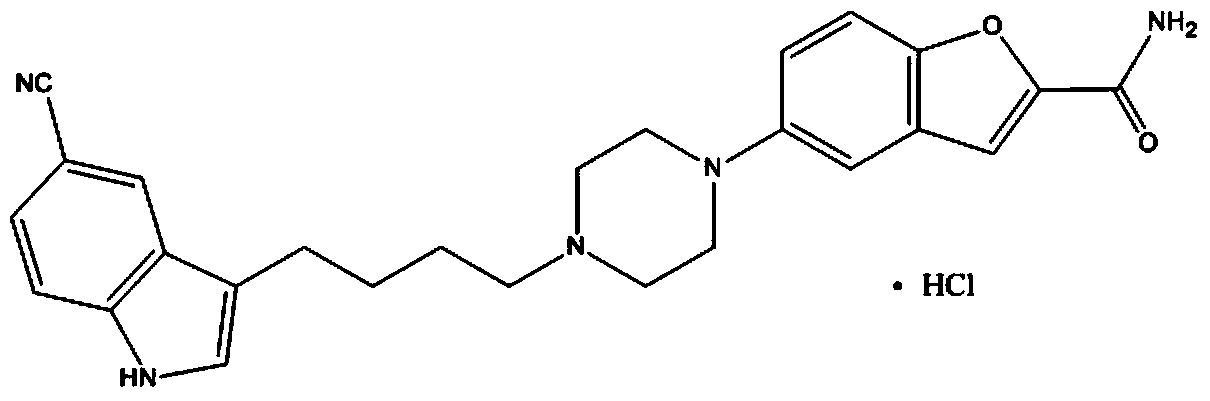
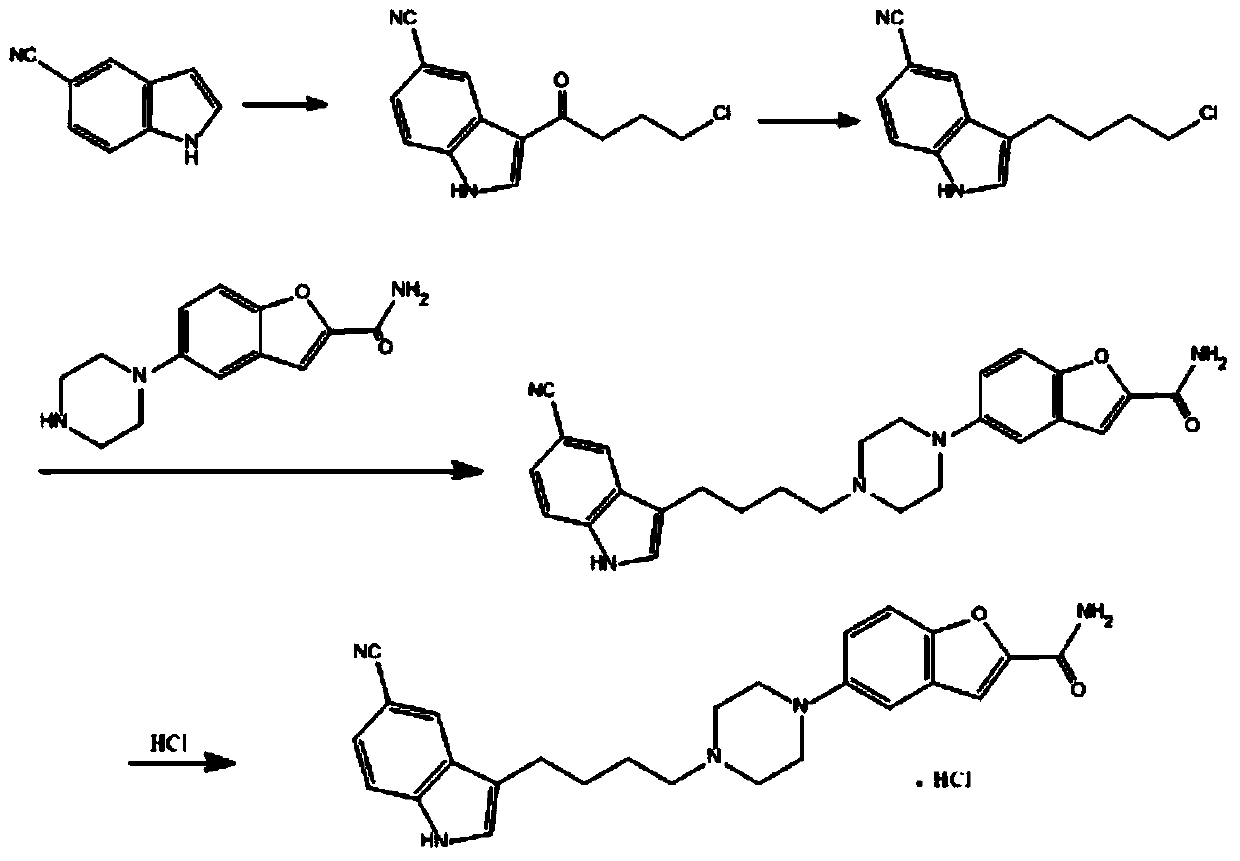
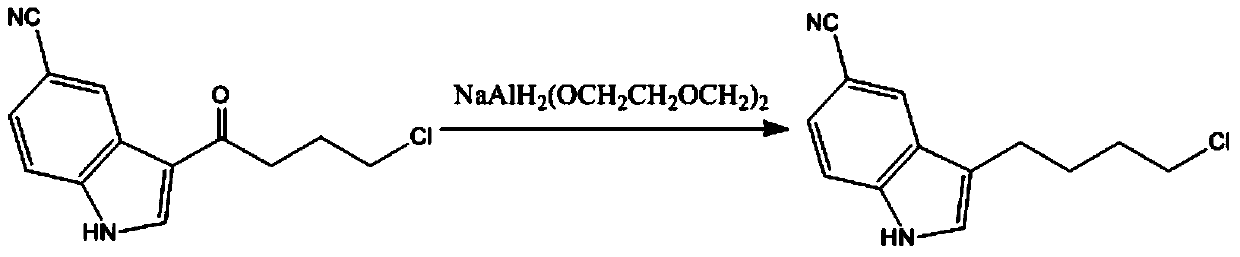Method for preparing vilazodone intermediate 3-(4-chlorobutyl)-1H-indol-5-cyano
A technology of vilazodone and intermediate, which is applied in the field of preparing vilazodone intermediate 3-(4-chlorobutyl)-1H-indole-5-cyano group, can solve the problem of high market price of sodium aluminate , not suitable for industrial amplification, not suitable for industrial amplification, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Add 3-(4-chlorobutyryl)-1H-indole-5-cyano (4.92g, 0.02mol) to a 100ml three-necked flask, and then add 60ml of tetrahydrofuran, stir to make the reaction raw materials fully dissolved in tetrahydrofuran , Then slowly add aluminum trichloride (5.32g, 0.04mol), use an ice bath to control the reaction temperature to 0-10 ℃, after all aluminum trichloride is added, slowly add potassium borohydride (2.16g, 0.04mol), always control the reaction temperature to 0-10°C, monitor the reaction with thin-layer chromatography, after the reaction is completed, slowly add water to the reaction flask to quench the reaction, control the temperature to 0-10°C, and use Extracted with dichloromethane, separated with a camera, washed the organic phase with water and saturated brine, concentrated in vacuo, evaporated to dryness, and recrystallized with acetone to obtain 4.42 g of the product with a yield of 95.05%.
[0029] MS(ESI + , M / e): 246.12[M+H] +
[0030] 1 H-NMR (400MHz, CDCl 3...
Embodiment 2
[0031] Example 2 Add 3-(4-chlorobutyryl)-1H-indole-5-cyano (4.92g, 0.02mol) to a 100ml three-necked flask, and then add 60ml of tetrahydrofuran, stir to make the reaction raw materials fully dissolved in tetrahydrofuran , Then slowly add ferric chloride (6.48g, 0.04mol, use an ice bath to control the reaction temperature to 0-10 ℃, after all ferric chloride is added, slowly add potassium borohydride (2.16g, 0.04mol) , Always control the reaction temperature to 0-10°C, monitor the reaction with thin layer chromatography, after the reaction is completed, slowly add water to the reaction flask to quench the reaction, control the temperature to 0-10°C, use dichloromethane after quenching After extraction and separation with a camera, the organic phase was washed with water and saturated brine, concentrated in vacuo and evaporated to dryness, and recrystallized with acetone to obtain 4.28 g of product with a yield of 92.04%.
Embodiment 3
[0032] Example 3 Add 3-(4-chlorobutyryl)-1H-indole-5-cyano (4.92g, 0.02mol) to a 100ml three-necked flask, and then add 60ml of tetrahydrofuran, stir to make the reaction raw materials fully dissolved in tetrahydrofuran , And then slowly add zinc chloride (5.44g, 0.04mol, use an ice bath to control the reaction temperature to 0-10 ℃, after all the zinc chloride is added, slowly add potassium borohydride (2.16g, 0.04mol) , Always control the reaction temperature to 0-10°C, monitor the reaction with thin layer chromatography, after the reaction is completed, slowly add water to the reaction flask to quench the reaction, control the temperature to 0-10°C, use dichloromethane after quenching After extraction and separation with a camera, the organic phase was washed with water and saturated brine, concentrated in vacuo and evaporated to dryness, and recrystallized with acetone to obtain 4.33 g of the product with a yield of 93.12%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com