Conjugated dienes derivative and preparation method thereof and application as anti-cancer drug
A technology of conjugated dienes and derivatives, which is applied in drug combination, antineoplastic drugs, organic chemistry, etc., can solve the problems of incomplete cure of cancer and prolonging the survival time of cancer patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
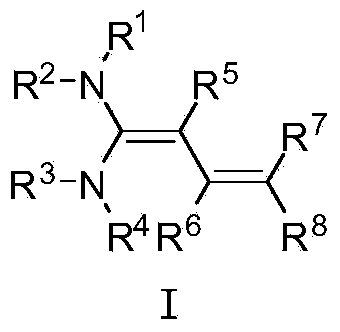
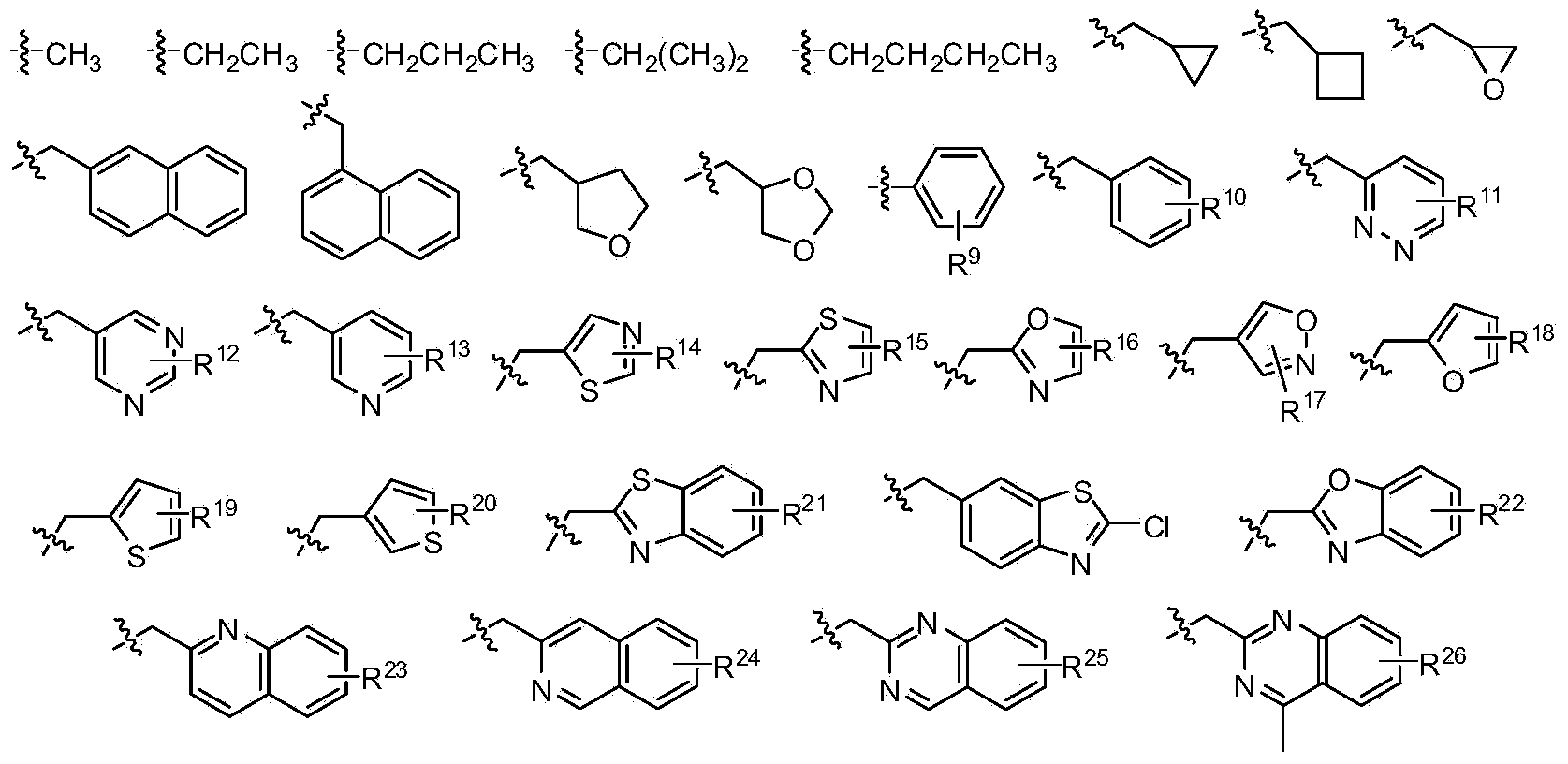
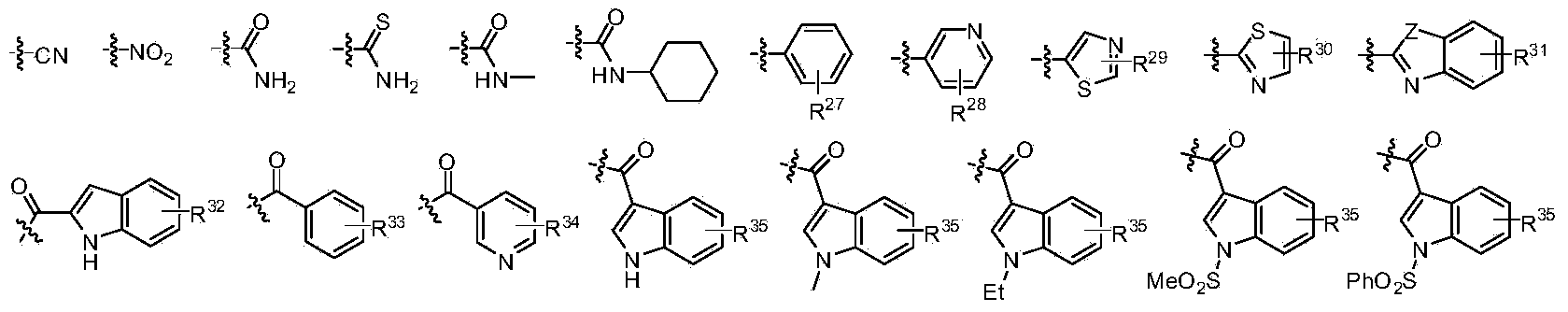
[0032] The structure of the partial conjugated diene derivative of the present invention is one of the specific compounds listed in Table 1:
[0033] Table 1: List of representative compound structures of general formula I
[0034]
[0035]
[0036]
[0037]
[0038]
Embodiment 2
[0040] Compound 2-(benzo[d]thiazol-2-yl)-4-(1-(phenyl)imidazoline-2-ylidene)-4-nitrobutyl- 2-alkene nitrile, its preparation method is:
[0041]
[0042] Using N-phenylethylenediamine as the starting material, referring to the method disclosed in the patent WO2004058714A1, successively undergoes a substitution reaction with 1,1-dithiomethyl-2-nitroethylene, and then reacts with N,N-dimethyl The intermediate 2-(1-(phenyl)imidazolin-2-ylidene)-2-nitroacetaldehyde (M-1) was prepared by condensation reaction of methyl formamide dimethyl acetal and alkaline hydrolysis reaction. Then weigh M-1, 0.12g (0.5mmol) of 2-(1-(phenyl)imidazolin-2-ylidene)-2-nitroacetaldehyde, dissolve it in 10mL absolute ethanol, and at room temperature, successively Add 0.096g (0.55mmol) of 2-cyanomethylbenzothiazole (compound A) and a catalytic amount of potassium hydroxide, stir the reaction mixture at room temperature for 30 minutes, then slowly heat to 40-45°C, keep warm for reaction, and monitor b...
Embodiment 3
[0044] Compound 2-(benzo[d]oxazol-2-yl)-4-(1-((6-chloropyridin-3-yl)methyl)imidazoline-2 numbered as I-37 in Example 1 -subunit)-4-nitrobut-2-enenitrile, its preparation method is:
[0045]
[0046] Using 2-chloro-5-chloromethylpyridine as the starting material, it undergoes the substitution reaction with ethylenediamine, the substitution reaction with 1,1-dithiomethyl-2-nitroethylene, and then with N,N -Dimethylformamide dimethyl acetal condensation reaction, alkaline hydrolysis reaction to prepare intermediate 2-(1-((6-chloropyridin-3-yl)methyl)imidazoline-2-ylidene)-2 - Nitroacetaldehyde (M-2). Then weigh M-2, namely 2-(1-((6-chloropyridin-3-yl)methyl)imidazolin-2-ylidene)-2-nitroacetaldehyde 0.14g (0.5mmol) and dissolve it in 10mL In absolute ethanol, at room temperature, 0.087 g (0.55 mmol) of 2-cyanomethylbenzoxazole (compound B) and a catalytic amount of piperidine were sequentially added, and the reaction mixture was first stirred at room temperature for 30 minute...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com