Preparation method of mesoporous zeolite molecular sieve
A mesoporous zeolite and molecular sieve technology, applied in the direction of crystalline aluminosilicate zeolite, borocarbane silicone crystalline aluminum silicate zeolite, etc., to achieve high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
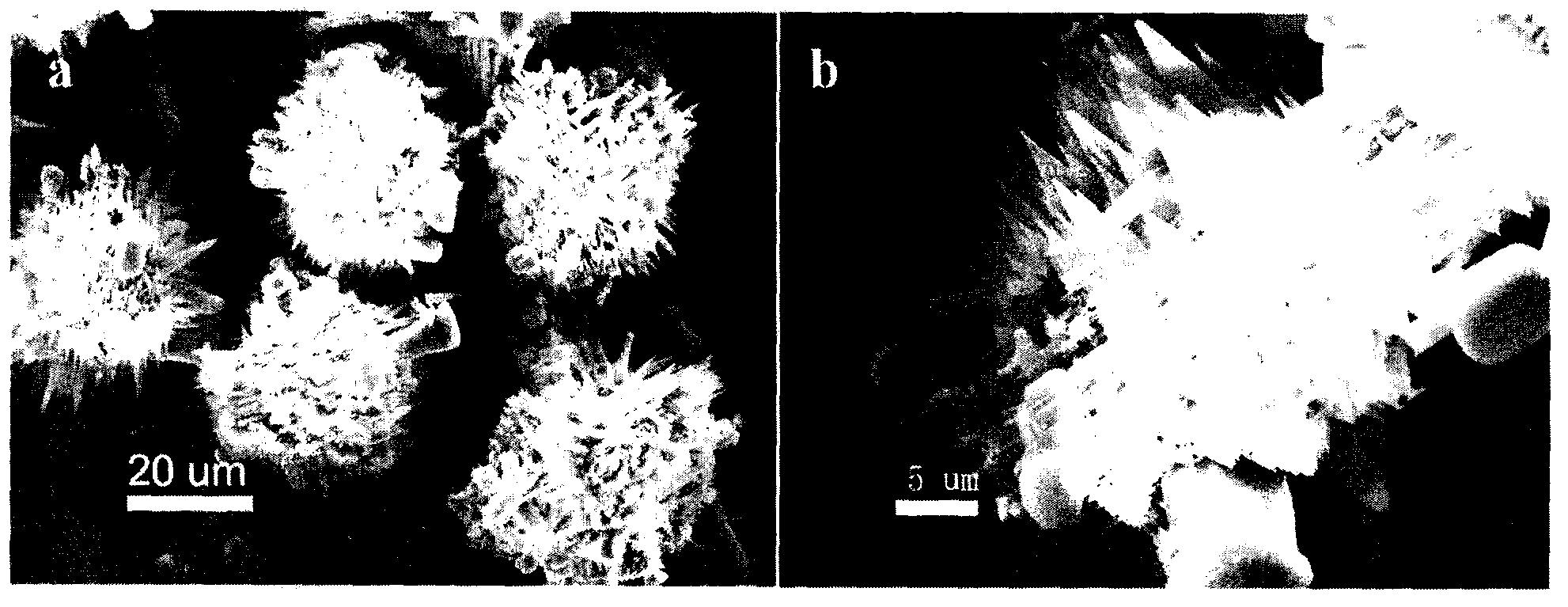
Image
Examples
Embodiment 1
[0019] Example 1: Synthesis of mesoporous silicalite-1 using a polyelectrolyte-surfactant complex as a template
[0020] Synthetic raw materials: polyacrylic acid (PAA), cetyltrimethylammonium bromide (CTAB), tetrapropylammonium hydroxide (TPAOH), tetraethylorthosilicate (TEOS), sodium chloride (NaCl), ten Octyldimethyl[trimethoxysilylpropyl]ammonium chloride (TPOAc).
[0021] Synthetic ratio: 10SiO 2 : 3.6TPAOH: 0.08TPOAc: 360H 2 O: 4.2NaCl
[0022] Synthesis steps: a certain amount of NaCl was dissolved in 5 ml of deionized water, and 25% TPAOH was added to form a clear solution. Add this solution to the previously synthesized CTAB and PAA complex, and stir for 20 minutes. TEOS and a small amount of TPOAc (50% in methanol) were added to the solution with stirring. Finally, stir at room temperature for 2 h to obtain a uniform mixed emulsion. Put it in a reaction kettle, and crystallize it with water at 140°C for 6 days. The obtained product was suction filtered, washed...
Embodiment 2
[0027] Example 2: Adding NaCl to synthesize mesoporous silicalite-1
[0028] Synthetic raw materials: polyacrylic acid (PAA), cetyltrimethylammonium bromide (CTAB), tetrapropylammonium hydroxide (TPAOH), tetraethylorthosilicate (TEOS), sodium chloride (NaCl), ten Octyldimethyl[trimethoxysilylpropyl]ammonium chloride (TPOAc).
[0029] Synthesis steps: directly add 25% TPAOH to the compound of CTAB and PAA, stir for 20min, the solution is still milky white. TEOS and a small amount of TPOAc (50% methanol solution) were added to the solution with stirring, and stirred for 30 min. Finally, dissolve a certain amount of NaCl in 5ml of water and add it to the above solution. Stir mechanically at room temperature for 2 h to obtain a viscous emulsion. Put it in a reaction kettle, and crystallize it with water at 140°C for 6 days. The obtained product was suction filtered, washed, dried at 80°C, and calcined at 550°C for 6h.
[0030] Mesoporous silicalite-1 was synthesized by using ...
Embodiment 3
[0033] Example 3: Synthesis of mesoporous silicalite-1 using other polyelectrolyte-surfactant complexes as templates
[0034] Synthetic raw materials: polyacrylic acid (PAA), cetylpyridinium chloride (CPC), tetrapropylammonium hydroxide (TPAOH), tetraethylorthosilicate (TEOS), sodium chloride (NaCl), octadecyl Dimethyl[trimethoxysilylpropyl]ammonium chloride (TPOAc).
[0035] Synthesis steps: the same as the steps of mesoporous silicalite-1 in Example 1, except that the cationic surfactant is changed into cetylpyridinium chloride (CPC).
[0036] The final effect of the mesoporous silicalite-1 synthesized by this method is not much different from the characterization in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com