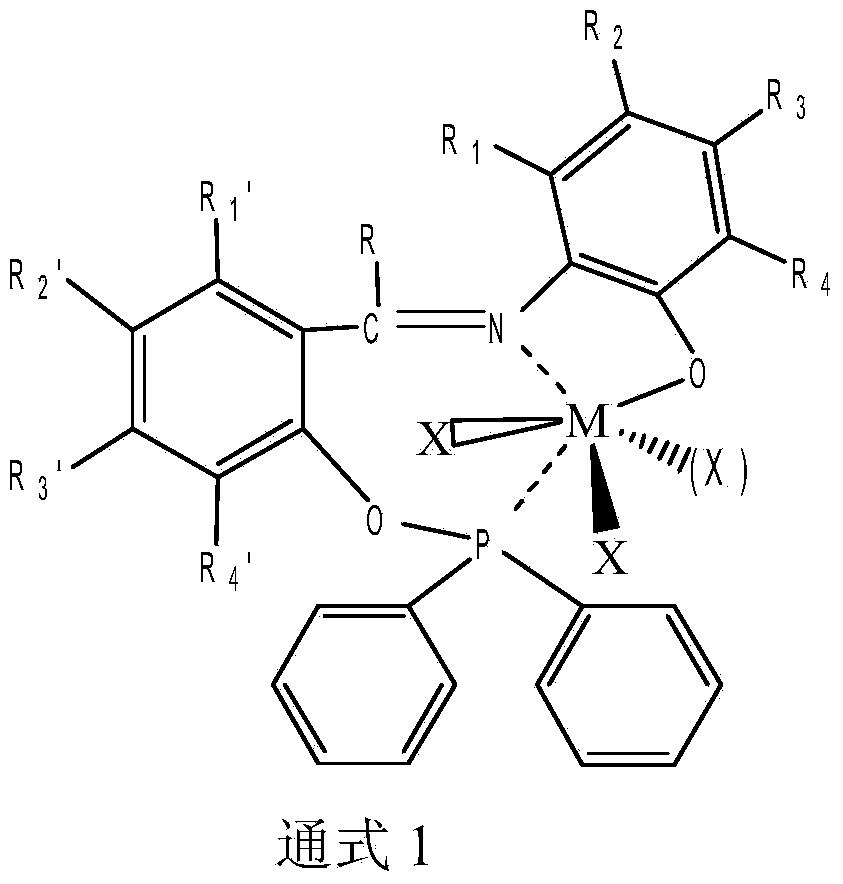
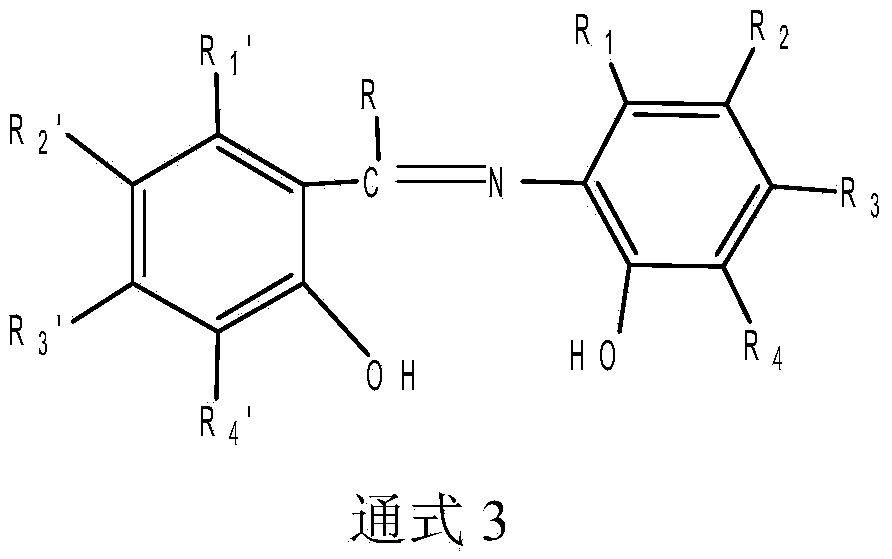
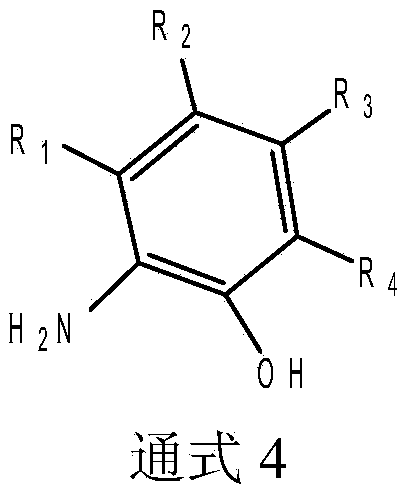
Bridge non-metallocene and preparation and application thereof
A non-metallocene, bridge-type technology, applied in the field of non-metallocene catalysts for olefin polymerization and olefin polymerization, can solve the problems of high cost, difficult structure modification, poor stability, etc., and achieve high insertion rate, high molecular weight, and good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] 1) Preparation of sodium salicylaldehyde phenate: under the protection of high-purity nitrogen, add 0.38g (9.5mmol) sodium hydroxide and 60mL absolute ethanol to a 300mL reaction flask, and stir to completely dissolve the sodium hydroxide in absolute ethanol . In this solution, add 1mL salicylaldehyde dropwise, react at room temperature for 3h, let stand, filter, wash 2-3 times with absolute ethanol, get 1.31g pale yellow powdered sodium salicylaldehyde phenate after draining, the yield 91.3%.
[0047] 2) Preparation of Schiff base sodium phenate: in a 300mL reaction bottle filled with high-purity nitrogen atmosphere, disperse 0.72g sodium salicylaldehyde phenate (5mmol) solid with absolute ethanol, stir, add 0.62g (5mmol) 2- Amino-4-methylphenol. At 80°C, reflux for 8h. Cool, let stand, filter, wash, and drain to obtain 0.65 g of red solid powder of Schiff base sodium phenolate, with a yield of 73.5%.
[0048] 3) Ligand preparation: Anhydrous and oxygen-free and un...
Embodiment 2
[0052] 1) Preparation of Schiff base sodium phenate: In a 300mL reaction flask filled with a high-purity nitrogen atmosphere, disperse 0.72g of sodium salicylaldehyde phenate (5mmol) solid with absolute ethanol, stir, and add 0.62g (5mmol) of 2- Amino-5-methylphenol. At 80°C, reflux for 8h. Cool, let stand, filter, wash, and drain to obtain 0.63 g of red solid powder of Schiff base sodium phenolate, with a yield of 71.8%.
[0053] 2) Preparation of the ligand: Anhydrous and oxygen-free and under the protection of high-purity nitrogen, take 0.55g of Schiff base sodium phenolate, disperse it in 30ml of toluene, and add 0.36ml (2mmol) of diphenyl to it dropwise at 0°C Phosphorus chloride was reacted for 2 hours, allowed to stand, filtered, washed, recrystallized, and vacuum-dried to obtain 0.46 g of ligand L2 with a yield of 33.5%.
[0054] 3) Preparation of non-metallocene main catalyst for olefin polymerization: under the protection of nitrogen atmosphere, take 0.20 g (0.8 mm...
Embodiment 3
[0057] 1) Preparation of Schiff base sodium phenate: In a 300mL reaction flask filled with high-purity nitrogen atmosphere, disperse 0.72g sodium salicylaldehyde phenate (5mmol) solid with absolute ethanol, stir, add 0.83g (5mmol) 2- Amino-4-tert-butylphenol. At 80°C, reflux for 8h. Cool, let stand, filter, wash, and drain to obtain 0.91 g of red solid powder, with a yield of 62.1%.
[0058] 2) Ligand preparation: Anhydrous and oxygen-free and under the protection of high-purity nitrogen, take 0.58g of Schiff base sodium phenolate, disperse it in 30ml of toluene, and add 0.36ml (2mmol) of diphenyl to it dropwise at 0°C Phosphorous chloride was reacted for 2 hours, allowed to stand, filtered, washed, recrystallized, and vacuum-dried to obtain 0.27 g of ligand L3, with a yield of 30.1%.
[0059] 3) Preparation of non-metallocene procatalyst for olefin polymerization: Under the protection of nitrogen atmosphere, 0.18g (0.4mmol) of ligand L3 was taken and dissolved in 30ml of to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com