A method for pure solid-phase synthesis of polypeptide antibiotic colistin
A solid-phase synthesis and resin synthesis technology, which is applied in the preparation methods of peptides, polymyxins, chemical instruments and methods, etc., can solve the problems of cumbersome steps and large amount of solvent used, so as to reduce the amount of use and simplify the synthesis process. and steps, the effect of shortening the composition time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
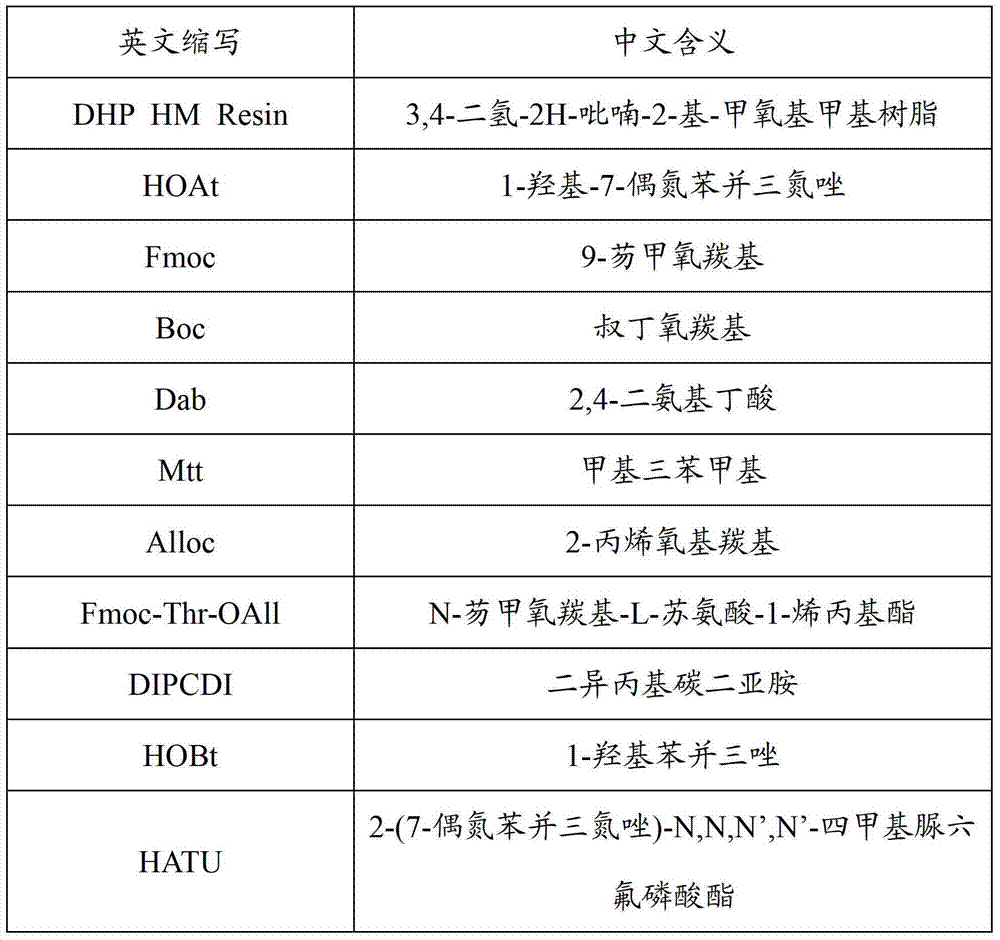
[0029] Embodiment 1: the preparation of the Fmoc-Thr(Resin)-OAll that the degree of substitution is 0.2mmol / g
[0030] Weigh 100g (100mmol) of DHP HM resin with a substitution degree of 1.0mmol / g, add it to a solid-phase reaction column, wash it twice with DCE, and swell the resin with DCE for 60 minutes, take 22.7g Fmoc-Thr-OAll (60mmol) and 7.5g PPTS (30mmol) was dissolved in 200ml DCM solution, added to the resin, and reacted at 80°C for 5h under the protection of nitrogen. After cooling and filtering, washing with DMF, DCM and Hexane three times respectively, and drying, the detected substitution degree was 0.202 mmol / g.
Embodiment 2
[0031] Embodiment 2: the preparation of the Fmoc-Thr(Resin)-OAll that the degree of substitution is 0.5mmol / g
[0032] Weigh 100g (100mmol) of DHP HM resin with a substitution degree of 1.0mmol / g, add it to a solid-phase reaction column, wash it twice with DCE, and swell the resin with DCE for 60 minutes, take 56.8g of Fmoc-Thr-OAll (150mmol) and 18.8g PPTS (75mmol) was dissolved in 200ml DCM solution, added to the resin, and reacted at 80°C for 5h under the protection of nitrogen. After cooling and filtering, washing with DMF, DCM and Hexane three times respectively, and drying, the detected substitution degree was 0.510mmol / g.
Embodiment 3
[0033] Embodiment 3: the preparation of the Fmoc-Thr(Resin)-OAll that the degree of substitution is 0.3mmol / g
[0034] Weigh 100g (100mmol) of DHP HM resin with a substitution degree of 1.0mmol / g, add it to a solid-phase reaction column, wash it twice with DCE, and swell the resin with DCE for 60 minutes, take 34.1g Fmoc-Thr-OAll (90mmol) and 11.3g PPTS (45mmol) was dissolved in 200ml DCM solution, added to the resin, and reacted at 80°C for 5h under the protection of nitrogen. After cooling and filtering, washing with DMF, DCM and Hexane three times respectively, and drying, the detected substitution degree was 0.308mmol / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com