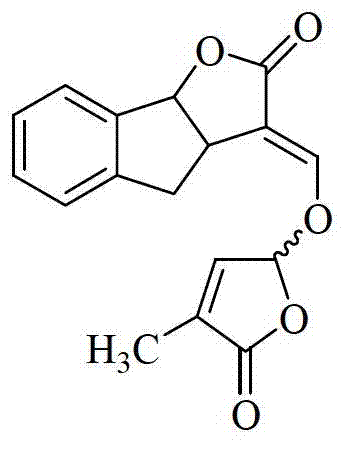
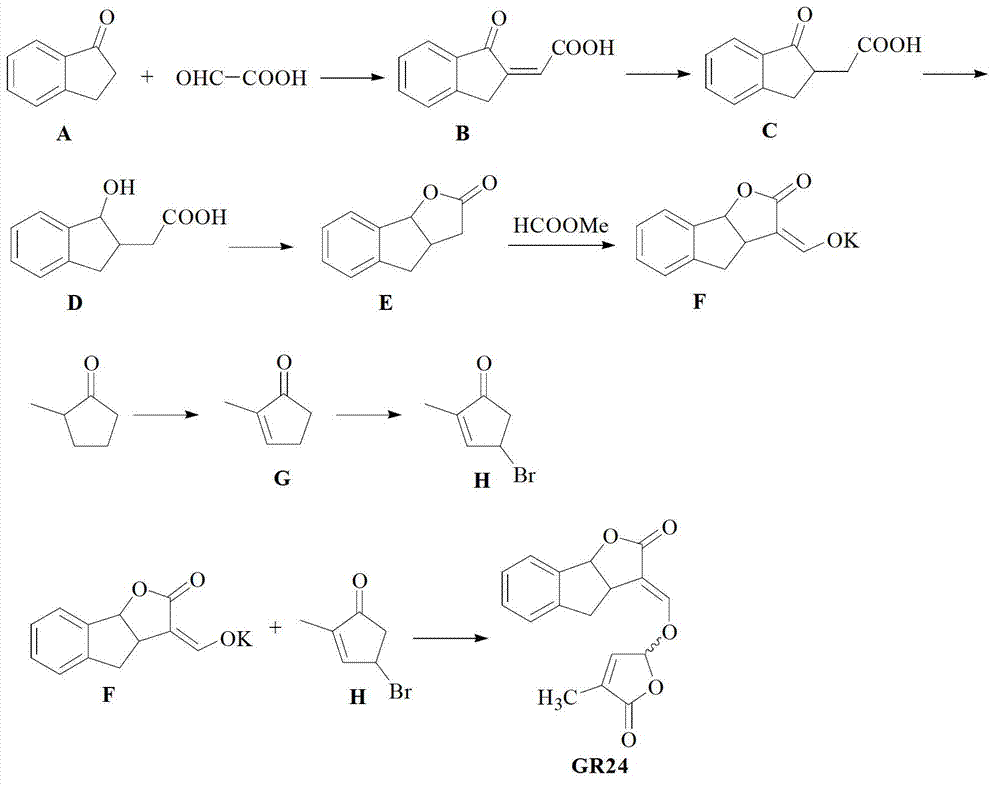
Total synthesis method of strigolactones GR24
A technology of strigolactone and GR24 is applied in the field of synthesizing a class of plant hormone strigolactones, which can solve the problems of lengthy synthesis methods, high toxicity or harsh operating conditions, and achieves high product purity, simple reaction conditions, The effect of cheap and easily available raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0037] Example: Preparation of strigolactone GR24 with 1-indanone as raw material
[0038] Synthesis of compound B: Add 1-indanone A (13.2 g, 0.1 mol), glyoxylic acid (17.5 g, 0.24 mol), tetrahydrofuran (20 ml) and concentrated hydrochloric acid (0.5 ml , 37%), heated to reflux for 1.5 hours, cooled to room temperature, added a large amount of cold water (200 mL) to precipitate the product, and obtained 18.63 g of light yellow solid Compound B, yield: 99%;
[0039] Synthesis of compound C: Add compound B (6.27 g, 0.033 mol), zinc powder (2.6 g, 0.04 mol), acetic acid (50 ml) and water (15 ml) into a single-necked flask (200 ml), and heat to reflux for 5 hours , after cooling to room temperature, the zinc powder was filtered off, the reaction solution was extracted with ethyl acetate, the organic phase was separated, and the organic phase was dried over anhydrous sodium sulfate, and concentrated to obtain 5.58 g of light yellow solid product Compound C, with a yield of 89%;
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com