Pharmaceutical composition containing tropisetron hydrochloride and fructose
A technology for tropisetron hydrochloride and tropisetron hydrochloride, which is applied in the field of medicine and can solve problems such as the occurrence of combined packaging of tropisetron hydrochloride injection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Preparation of Tropisetron Hydrochloride for Injection
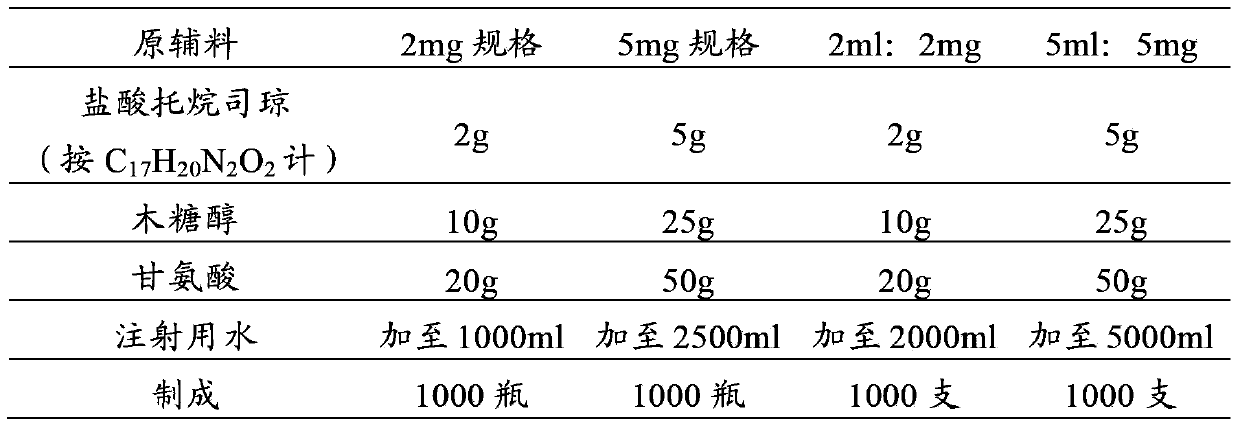
[0039] prescription:
[0040]
[0041]
[0042] Preparation Process:
[0043] (1) First add 2800ml of water for injection into the container, and control the temperature at 10°C to 20°C;
[0044] (2) Add 35g xylitol and 70g glycine, stir to dissolve completely;
[0045] (3) Add 7g of tropisetron hydrochloride (press C 17 h 20 N 2 o 2 meter), stir to dissolve completely, and adjust the pH to 4.8 with 1mol / L sodium hydroxide solution or 1mol / L hydrochloric acid solution;
[0046] (4) Add 0.7g of activated carbon for injection, add the remaining water for injection, set the volume to 3500ml, stir and absorb for 30 minutes;
[0047] (5) The solution is decarbonized by coarse filtration, coarsely filtered through a 0.45 μm cartridge filter, and then sterilized and filtered with a 0.22 μm microporous membrane until the visible foreign matter is qualified;
[0048] (6) Filling.
[0049] (7) Free...
Embodiment 2
[0058] Example 2 Preparation of Tropisetron Hydrochloride Injection
[0059] prescription:
[0060]
[0061] Preparation Process:
[0062] (1) First add 5600ml of water for injection into the container, and control the temperature at 10°C to 20°C;
[0063] (2) Add 35g xylitol and 70g glycine, stir to dissolve completely;
[0064] (3) Add 7g of tropisetron hydrochloride (press C 17 h 20 N 2 o 2 meter), stir to dissolve completely, and adjust the pH to 5.1 with 1mol / L sodium hydroxide solution or 1mol / L hydrochloric acid solution;
[0065] (4) Add 1.4g of activated carbon for injection, add the remaining water for injection, set the volume to 7000ml, stir and absorb for 30 minutes;
[0066] (5) The solution is decarbonized by coarse filtration, coarsely filtered through a 0.45 μm cartridge filter, and then sterilized and filtered with a 0.22 μm microporous membrane until the visible foreign matter is qualified;
[0067] (6) Filling, sealing, and sterilization.
Embodiment 3
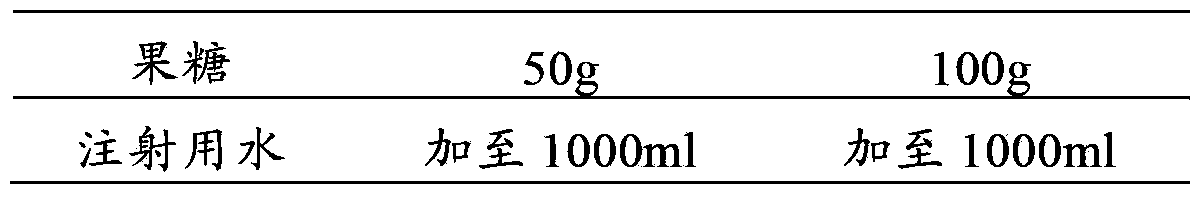
[0072] Example 3 Preparation of Fructose Injection
[0073] prescription:
[0074]
[0075] Preparation Process:
[0076] (1) Concentrated formulation: Add 30L of water for injection into the concentrated formulation tank, add 10,000g of fructose, stir to dissolve, add 100g of wet medicinal charcoal, stir for 30 minutes, filter and decarbonize, filter into the diluted formulation tank, Rinse the concentrated preparation tank with water for injection several times, and filter into the thin preparation tank;
[0077] (2) Dilute formulation: add 90L of water for injection to the dilute formulation tank, adjust the pH value to 4.0 with 0.1mol / L hydrochloric acid solution, add 100g of wet medicinal charcoal, stir for 30 minutes, filter and decarbonize, Add water for injection to 100L;
[0078] (3) Fine filter with 0.22μm microporous membrane, fill, 250ml / bottle and 500ml / bottle, seal;
[0079] (4) Sterilization: Sterilize by autoclaving at 121°C for 15 minutes, quickly spr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com