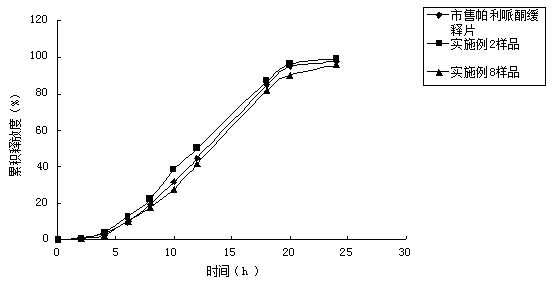
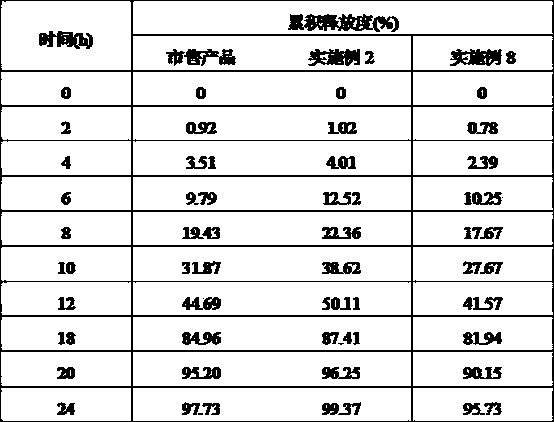
Pharmaceutical composition of palipeddone
A kind of technology of paliperidone and composition, applied in the field of antipsychotic drugs, the pharmaceutical composition field of paliperidone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Ball core:
[0019] components Dosage (g) Paliperidone 3.0 lactose 100.0 sucrose 20.0 HPMC 60.0
[0020] Coating layer:
[0021] components 100 servings (g) Udrake RL100 3.0 Udrake RS100 6.0 PEG4000 1.8 60% ethanol 89.2
[0022] Preparation:
[0023] Add an appropriate amount of wetting agent according to the prescription amount of the pellet core and mix it to make a wet material, extrude the strip through a sieve plate, and make a pellet core in a spheronizer. After the pellet core is dried, it is coated on a fluidized bed to obtain pellets, and the weight gain of the coating is 10%.
Embodiment 2
[0025] Ball core:
[0026] components Dosage (g) Paliperidone 3.0 microcrystalline cellulose 36.0 lactose 90.0
[0027] Isolation layer:
[0028] components Dosage (g) HPMC-E5LV 6.0 PEG400 1500 talcum powder 5 60% ethanol 8.0
[0029] Coating layer:
[0030] components 100 servings (g) Udrake RL100 6.0 Udrake RS100 3.0 PEG4000 1.8 60% ethanol 89.2
[0031] The preparation method is the same as in Example 1.
Embodiment 3
[0033] Ball core:
[0034] components Dosage (g) Paliperidone 6.0 lactose 160.0 starch 42.0 EC 80.0 Eudragi RSPO 120.0
[0035] Coating layer:
[0036] components 100 servings (g) Udrake RL100 1.0 Udrake RS100 9.0 PEG4000 2.0 60% ethanol 88.0
[0037] The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com