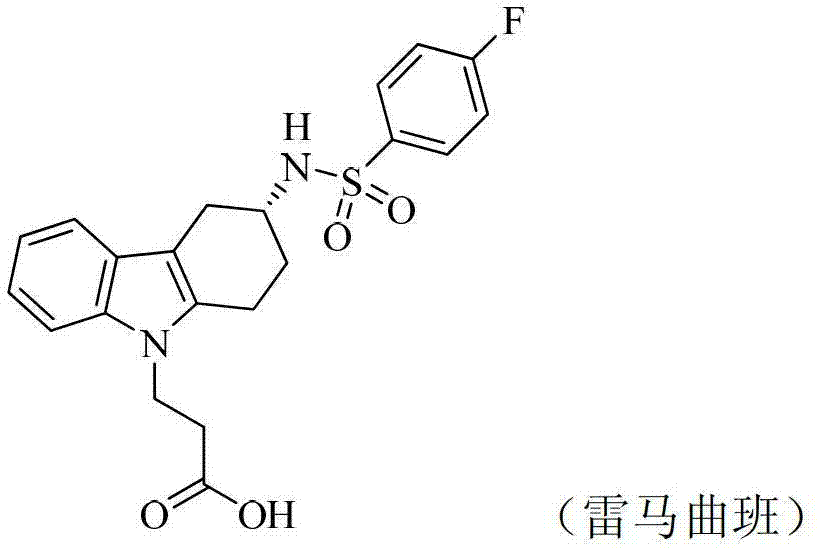
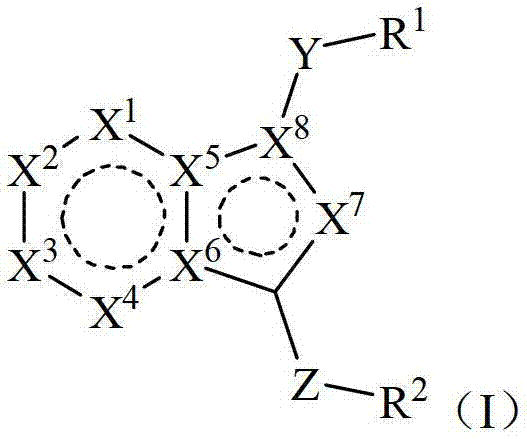
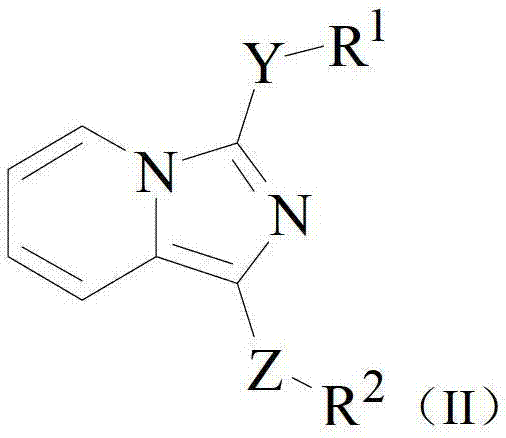
Bicyclic derivatives serving as CRTH2 receptor antagonist
A compound, cycloalkyl technology, applied in the field of bicyclic derivatives as CRTH2 receptor antagonists, can solve the problems of prolonged thromboplastin time, poor selectivity, weak CRTH2 receptor antagonistic effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] Example 1 Preparation of 2-[1-(4-chlorophenylthio)imidazo[1,5-a]pyridin-3-yl]acetic acid (Compound 1)
[0122]
[0123] (1) Ethyl malonate monoyl chloride
[0124] Ethyl malonate (13.2g, 100.0mmol) and N,N-dimethylformamide (0.05mL) were stirred and dissolved in dichloromethane (200mL), cooled to 0°C in an ice-water bath, and oxalyl chloride ( 25.4g, 200mmol), the mixture was stirred at room temperature for 2h, and concentrated to obtain ethyl malonyl chloride (14.5g, 97%) as a white oil.
[0125] (2) Ethyl 3-oxo-3-(pyridin-2-ylmethyleneamino)propionate
[0126]
[0127] Ethyl malonyl chloride (14.5g, 96.7mmol) was dissolved in dichloromethane (150mL), cooled to 0°C in an ice-water bath, and triethylamine (19.5g, 193mmol) dissolved in 50mL of dichloromethane was added dropwise and 2-aminomethylpyridine (13.6g, 125.7mmol), stirred at room temperature for 3 hours. Then the solvent was removed by rotary evaporation, and the residue was separated by silica gel colu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com