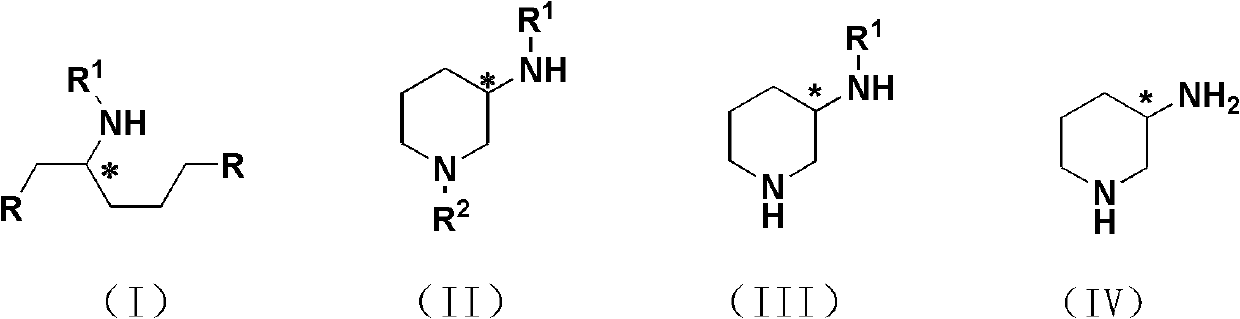
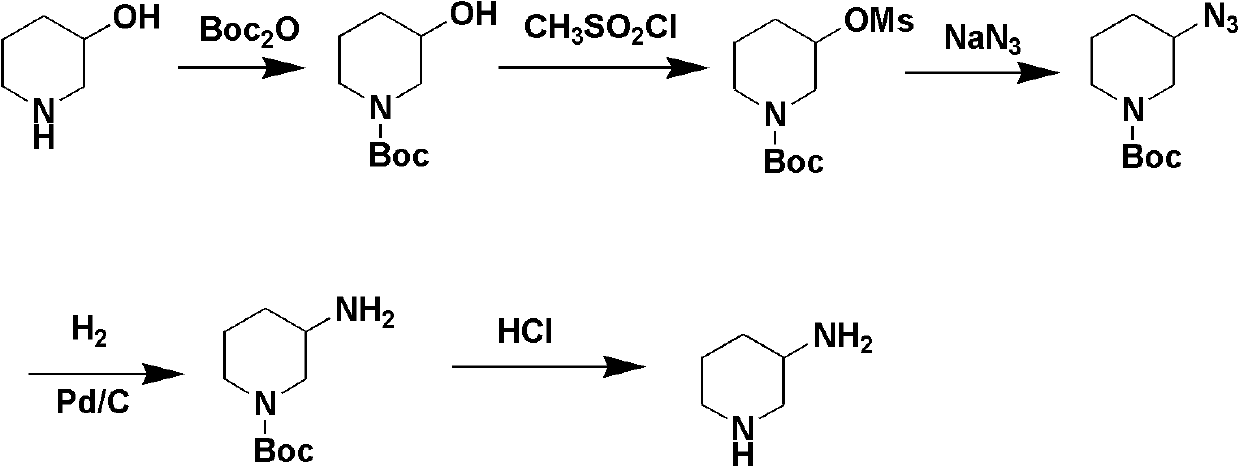
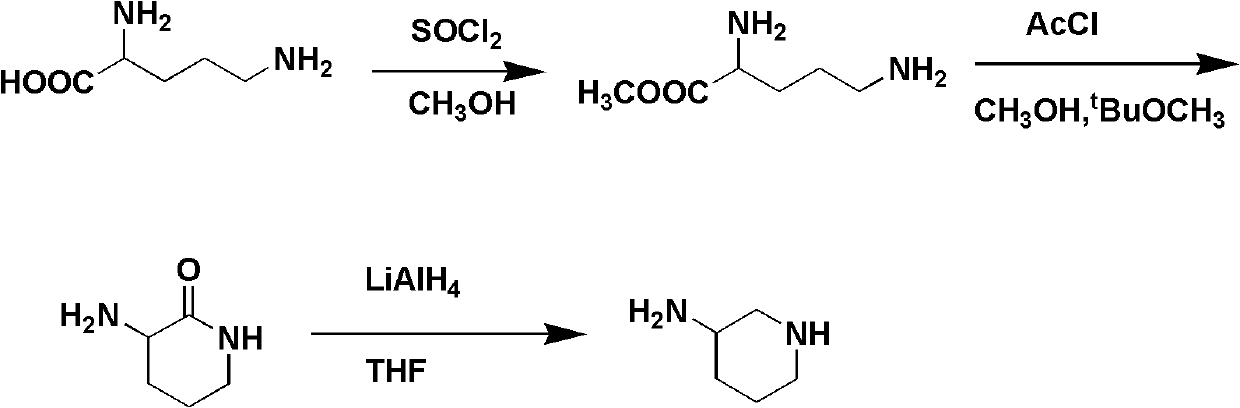
Preparation method for 3-aminopiperidine and optical isomer thereof
A technology of optical isomers and aminopiperidine, which is applied in the field of preparation of heterocyclic compounds, can solve the problems of cumbersome synthesis process and achieve the effects of high optical purity, mild reaction conditions and less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 1. Preparation of (R)-1-allyl-3-(benzyloxycarbonylamino)piperidine
[0044] Weigh allylamine (22.55g, 0.41mol) in a one-necked bottle, and add (R)-2-((benzyloxycarbonyl)amino)pentane-1,5-dimesylate [Heterocycles, 44(1), 213-225, 1997; Syn.Comm.28(21), 3919-3926, 1998; Eur.J.Org.Chem., (9), 1765-1776, 2005] (40.9g, 0.1 mol), the added solid dissolves slowly, after adding, heat up to 50°C and stir, and a large amount of solid (allylamine methanesulfonate) appears instantly after about 1h, stop stirring, keep warm for 30min, add ethyl acetate to dissolve the product, and heat up to Stir at 70°C until it is evenly stirred, filter off the solid, wash the organic phase three times with water, dry, filter, spin dry to obtain a yellow solid, recrystallize to obtain 22.3g of a white solid, which is (R)-1-allyl-3 -(Benzyloxycarbonylamino)piperidine (yield 81.3%).
[0045] 1 H-NMR (CDCl3, 400MHz): δ1.53-1.69(m, 4H), 2.23-2.45(m, 4H), 2.93(d, 2H), 3.78-3.81(m, 1H), 5.06(s, 2H) ...
Embodiment 2
[0059] 1. Preparation of (R)-1-(4-methylbenzyl)-3-(benzyloxycarbonylamino)piperidine
[0060] Weigh 4-methylbenzylamine (49.61g, 0.41mol) in a single-necked bottle, add (R)-2-((benzyloxycarbonyl)amino)pentane-1,5-dimethylsulfonic acid in batches under stirring Esters (40.9g, 0.1mol), the added solids were slowly dissolved, after the addition was complete, the temperature was raised to 50°C and stirred, and a large amount of solids (4-methylbenzylamine methanesulfonate) appeared instantly after about 1h, the stirring was stopped, and the temperature was kept for 30min , add ethyl acetate to dissolve the product, heat up to 70°C and stir until evenly stirred, filter off the solid, wash the organic phase three times with water, dry, filter, spin dry to obtain a yellow solid, recrystallize to obtain 30.5g of a white solid, as ( R)-1-(4-methylbenzyl)-3-(benzyloxycarbonylamino)piperidine (yield 90.3%).
[0061] 1 H-NMR (CDCl 3 , 400MHz): δ1.89-1.98(m, 2H), 2.00-2.05(m, 2H), 2.35(...
Embodiment 3
[0066] 1. Preparation of (R)-1-(4-methylbenzyl)-3-(tert-butoxycarbonylamino)piperidine
[0067]Weigh 4-methylbenzylamine (49.61g, 0.41mol) in a single-necked bottle, add (R)-2-((tert-butoxycarbonyl)amino)pentane-1,5-dimethylsulfonate in batches under stirring Ester (37.5g, 0.1mol), the added solid dissolved slowly, after the addition was completed, the temperature was raised to 50°C and stirred, and a large amount of solid (4-methylbenzylamine methanesulfonate) appeared instantly after about 1h, the stirring was stopped, and the temperature was kept After 30 minutes, add ethyl acetate to dissolve the product, raise the temperature to 70°C and stir until it is evenly stirred, filter off the solid, wash the organic phase three times with water, dry, filter, and spin dry to obtain a yellow solid, which is recrystallized to obtain 27.1g of a white solid. (R)-1-(4-methylbenzyl)-3-(tert-butoxycarbonylamino)piperidine (yield 89.4%).
[0068] 1 H-NMR (CDCl 3 , 400MHz): δ1.41(s, 9H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com