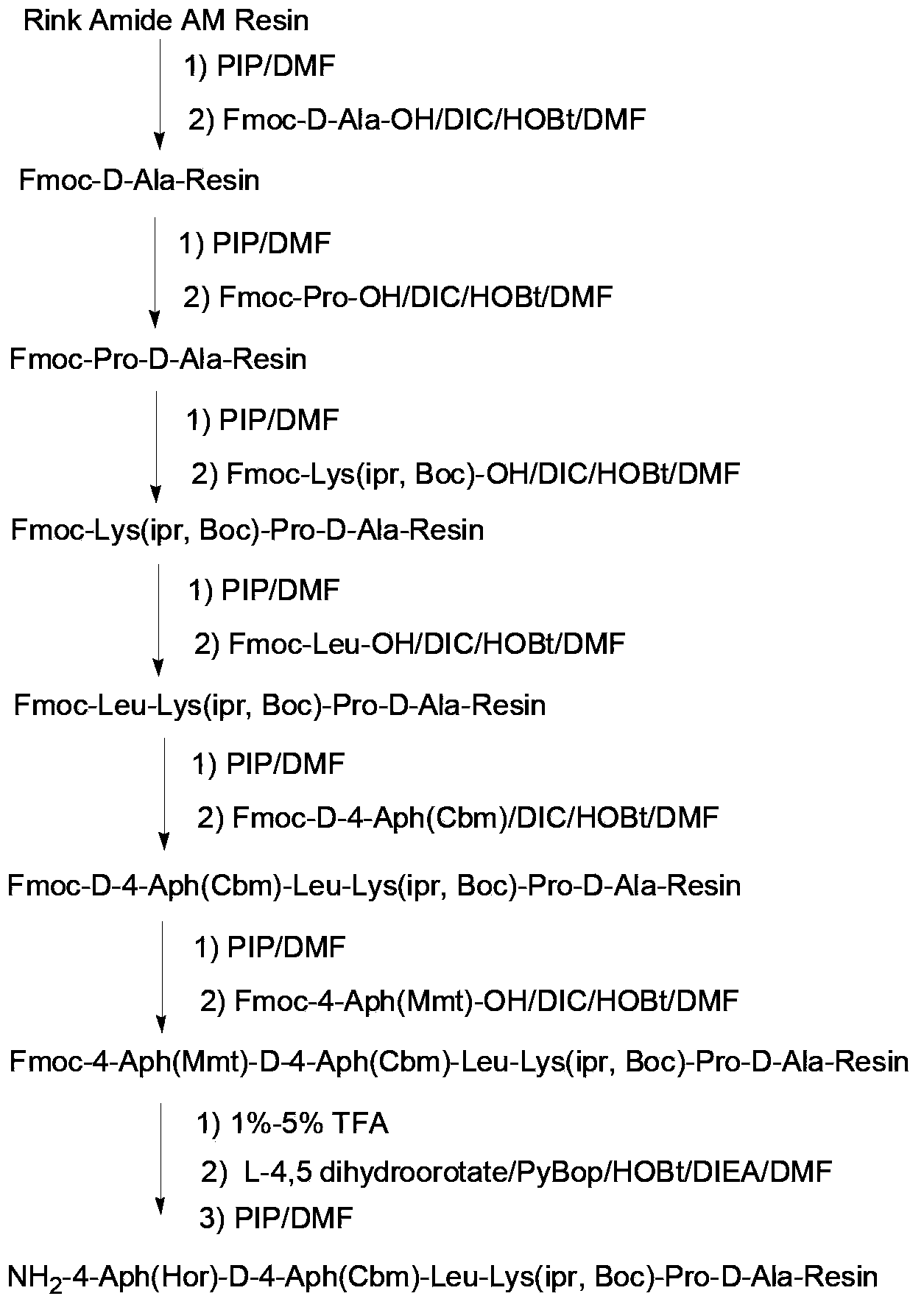
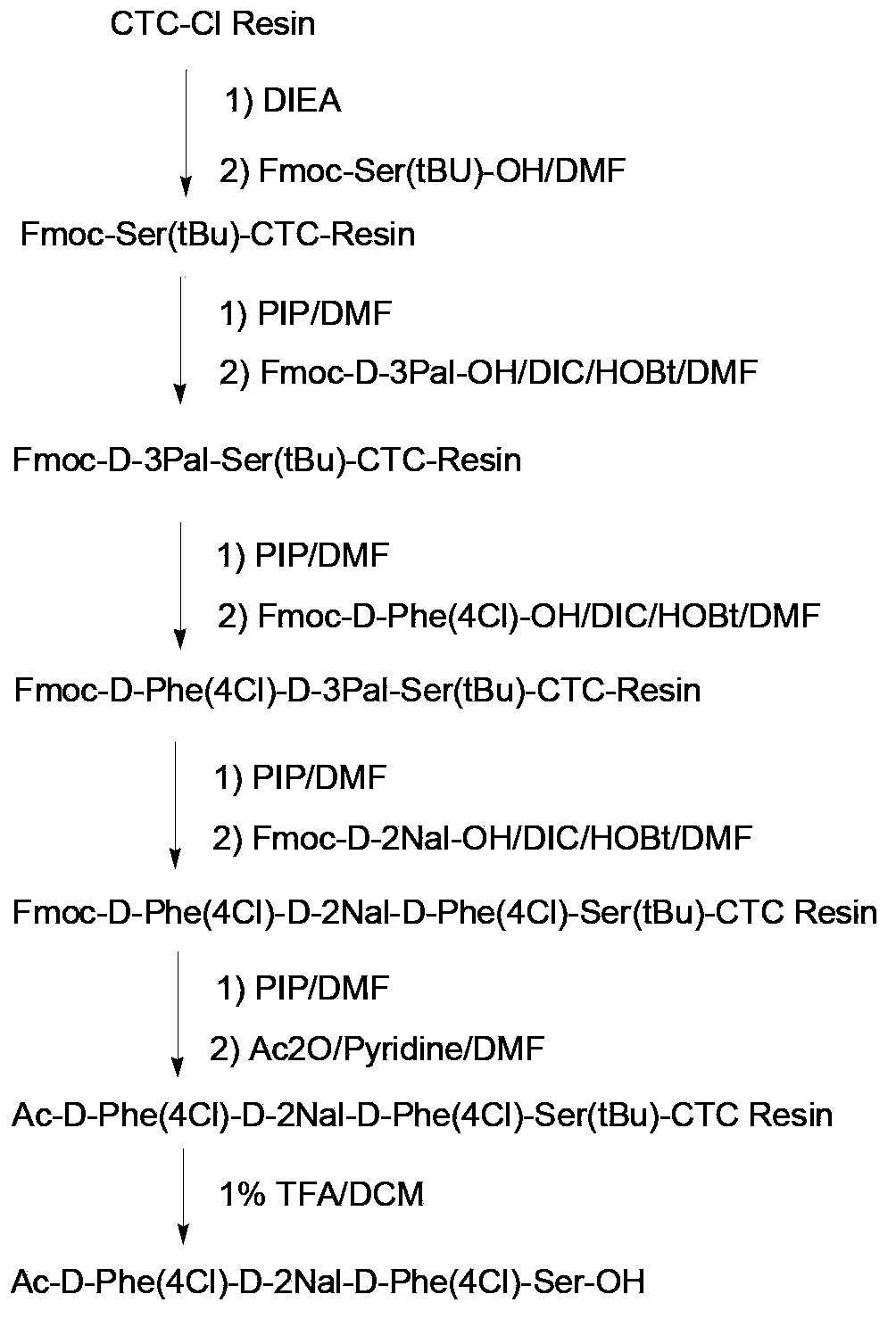
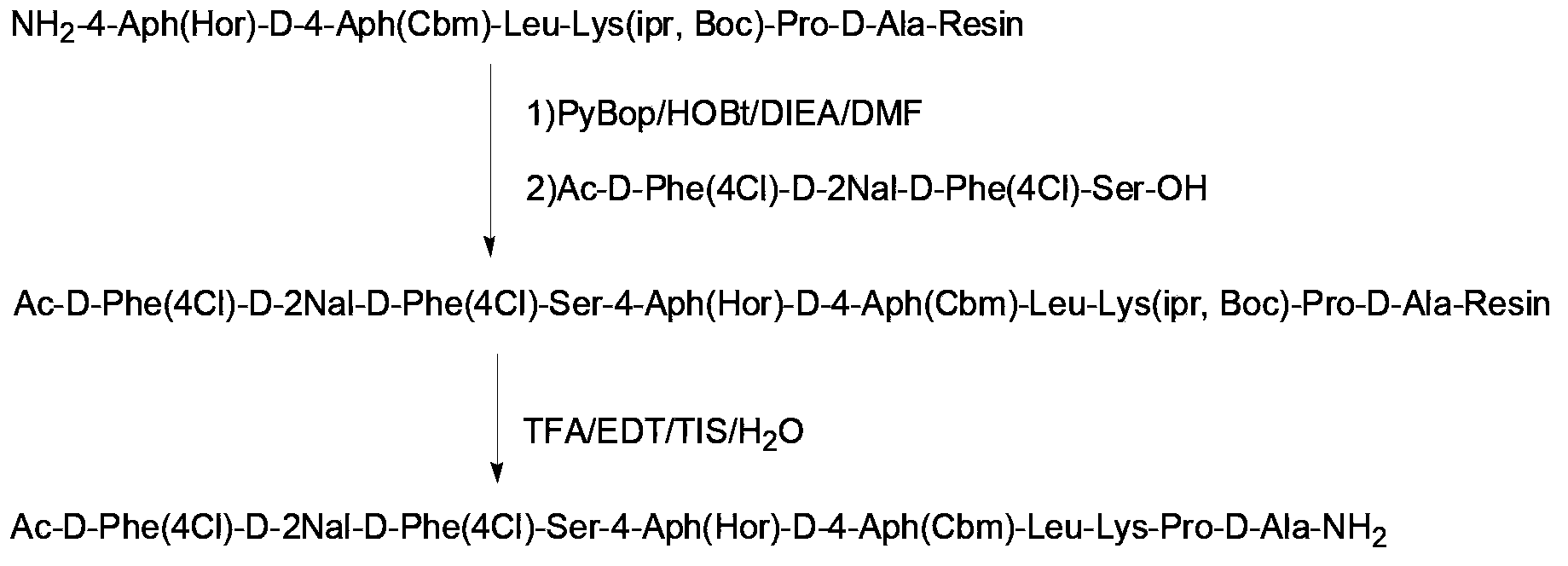
Synthesis of degarelix by solid phase segment method
A technology of solid-phase synthesis and degarelix, applied in peptide preparation methods, chemical instruments and methods, organic chemistry, etc., can solve problems such as high price, shedding, and increased cost of large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] Partial structural formulas involved in the preparation method provided by the invention are shown in the following table:
[0043]
[0044] As used herein, "solid phase synthesis" or "solid phase peptide synthesis (solid phase peptide synthesis)" is a peptide synthesis technique well known in the art, including but not limited to the following methods: covalently synthesize an amino-protected amino acid Linked (bonded) on a solid-phase support; in the presence of a deprotecting agent, the protective group of the amino group is removed, so that the first amino acid is connected to the solid-phase support; then the amino group is blocked (protected) by the second amino acid The carboxyl group is activated, and the second amino acid activated by the carboxyl group reacts (condenses) with the amino group of the first amino acid that has been attached to the solid-phase support to form a peptide bond, thus forming a protective group on the solid-phase support. Dipeptide:...
Embodiment 1
[0108] Synthesis of Fmoc-D-Ala-AM resin
[0109] Add Rink Amide-AM resin (5mmol, substitution degree 0.5mmol / g) into the solid phase reactor, add 100ml DMF to swell for 30min, and wash twice with DMF. Add 100ml of 20% PIP / DMF solution to remove Fmoc protection for 10 minutes, drain, then add 100ml of 20% PIP / DMF solution to remove Fmoc protection for 20 minutes, wash with DMF for 3 times, DCM for 2 times, and DMF for 3 times. 4.67g Fmoc-D-Ala-OH (15.0mmol, 3.0eq.) and 3.04g HOBt (22.5mmol, 4.5eq.) were dissolved in 80ml DMF, and 4.7ml DIC (30.0mmol, 6.0eq.) was added at 0-5°C .), pre-activated for 2-5 minutes, added to the solid-phase reactor, and reacted with nitrogen gas for 2-3 hours, and the ninhydrin test was negative. Drained, washed 3 times with DMF and 3 times with DCM, and dried to obtain Fmoc-D-Ala-AM resin, the degree of substitution was 0.42mmol / g.
Embodiment 2
[0111] Synthesis of Fmoc-D-Ala-MBHA Resin
[0112] Add Rink Amide-MBHA resin (5mmol, substitution degree 0.8mmol / g) into the solid phase reactor, add 100ml DMF to swell for 30min, and wash twice with DMF. Add 100ml of 20% PIP / DMF solution to remove Fmoc protection for 10 minutes, drain, then add 100ml of 20% PIP / DMF solution to remove Fmoc protection for 20 minutes, wash 3 times with DMF, 2 times with DCM, and 2 times with DMF. Dissolve 4.67g Fmoc-D-Ala-OH and 3.04g HOBt in 80ml DMF, add 4.7ml DIC at 0-5°C, pre-activate for 2-5min, add it to a solid-phase reactor, react with nitrogen gas for 2-3h, ninhydrin The ketone test was negative. Drained, washed 3 times with DMF and 3 times with DCM, and dried to obtain Fmoc-D-Ala-MBHA resin, the degree of substitution was 0.58mmol / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com