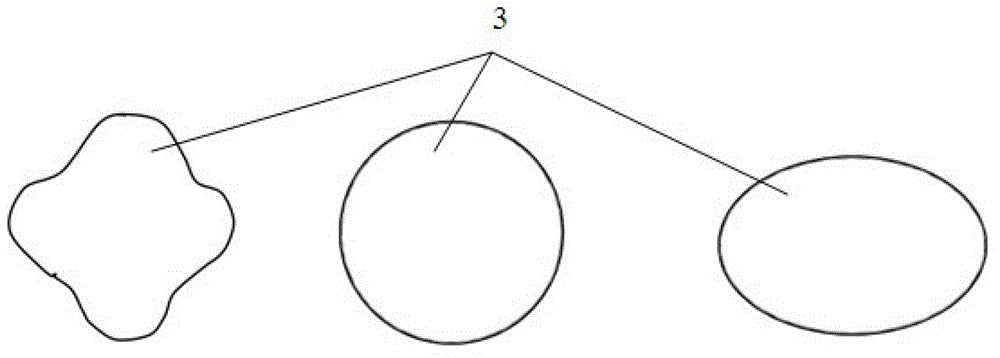
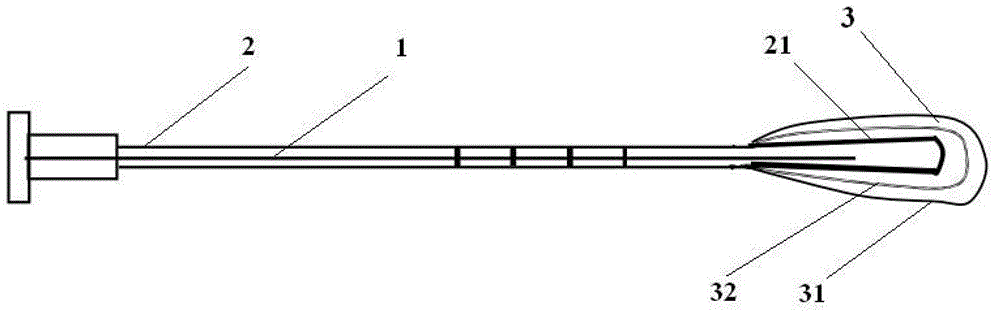
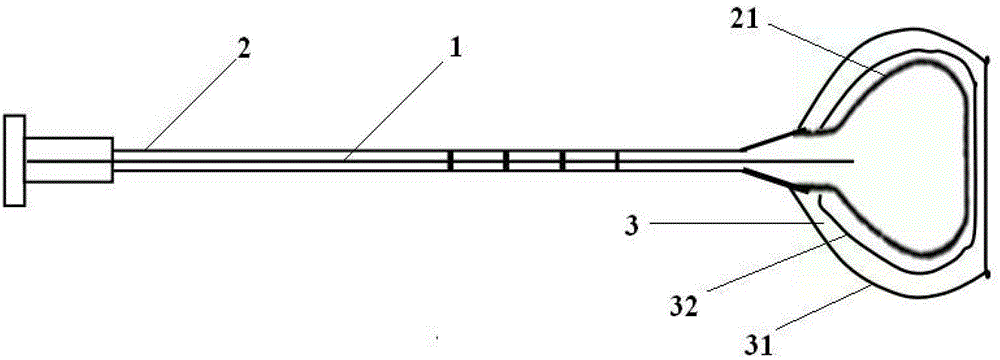
Degradable compound active amnion material, preparation method and application thereof and compound active amnion uterine cavity repair stent
An amniotic and active technology, which is applied in the field of composite active amniotic membrane uterine cavity repair, can solve the problems of accelerated postoperative adhesion, secondary damage of metal stents, and poor effect, and achieves strong operability, avoids secondary damage, and protects the human body. good adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] 1. Preparation of composite active amniotic membrane material
[0031] 1.1 Preparation of amniotic membrane
[0032] The amniotic membrane in this embodiment adopts human amniotic membrane, and the human amniotic membrane can be selected from commercial human amniotic membranes that meet the standard (YZB / Guo 0593-2005). During the treatment of the amniotic membrane, the amniotic membrane epithelial cells can be retained or the epithelial cells can be removed by enzymatic hydrolysis , no matter whether the epithelial cells are removed or not, the preparation of the composite active amniotic membrane material in the present invention is not affected. Among them, the methods are as follows:
Embodiment 1
[0034] Preparation of amniotic membrane retaining epithelial cells: In a clean and sterile environment, fresh amniotic membranes were rinsed with sterile saline, with the epithelial layer facing upward, and were flattened on 0.45um microporous nitrocellulose filter paper, placed in 0.2% ~ Soak in 2% glycerol for later use.
Embodiment 2
[0036] Preparation of amniotic membrane without epithelial cells: in a clean and sterile environment, fresh amniotic membrane is rinsed with sterile water, and then treated with 0.2% to 0.5% trypsin or 0.25% trypsin + 0.02% EDTA (ethylenediaminetetraacetic acid, Ethylene diamine tetraacetic acid), hydrolyzed by shaking in a water bath at 37°C for 20 to 120 minutes, remove epithelial cells, fibroblasts and serum with a cell scraper, and rinse with sterile water repeatedly. At this time, the decellularized amniotic membrane is a translucent membrane, HE Staining (hematoxylin-eosin staining), no epithelial cell residues were observed under light microscope, and soaked in 0.2%-1% glycerol for later use.
[0037] 1.2 Preparation of collagen sponge or composite collagen sponge preparation solution
[0038] The present invention includes two preparation methods of collagen sponges. The collagen sponges or composite collagen sponges prepared by the following methods are suitable for t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com