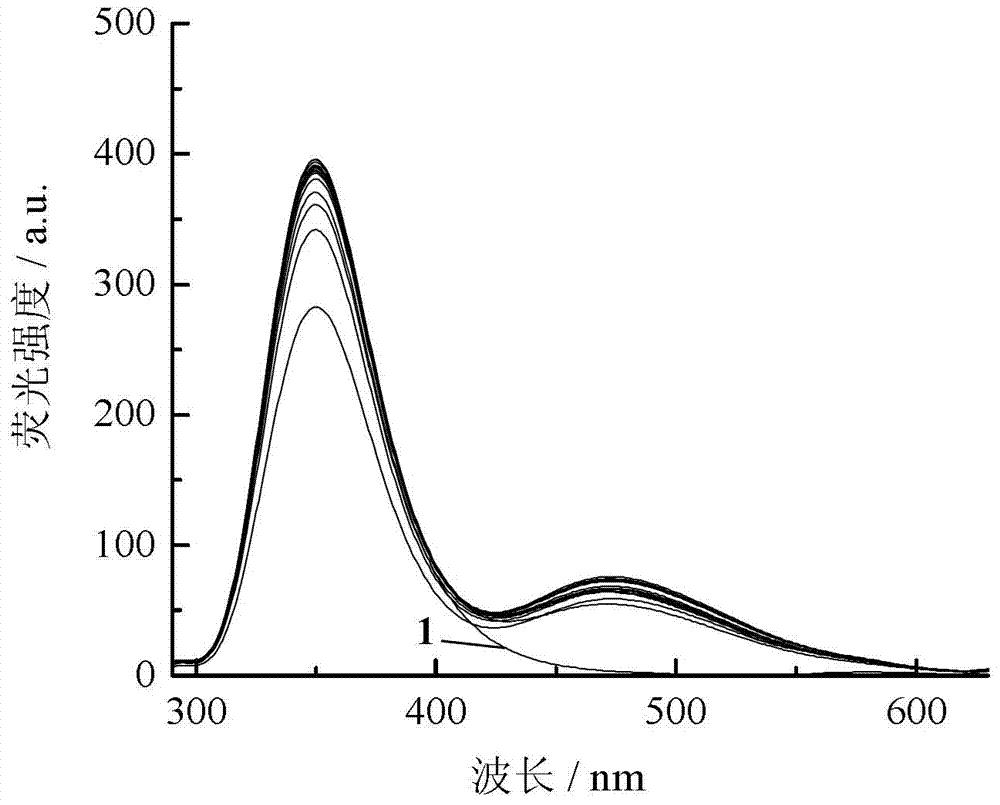
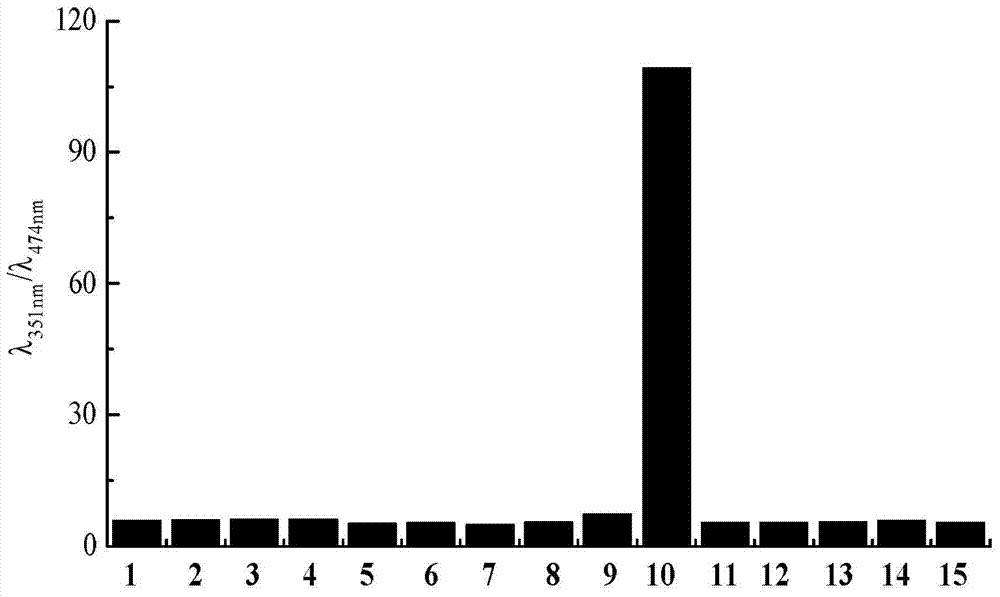
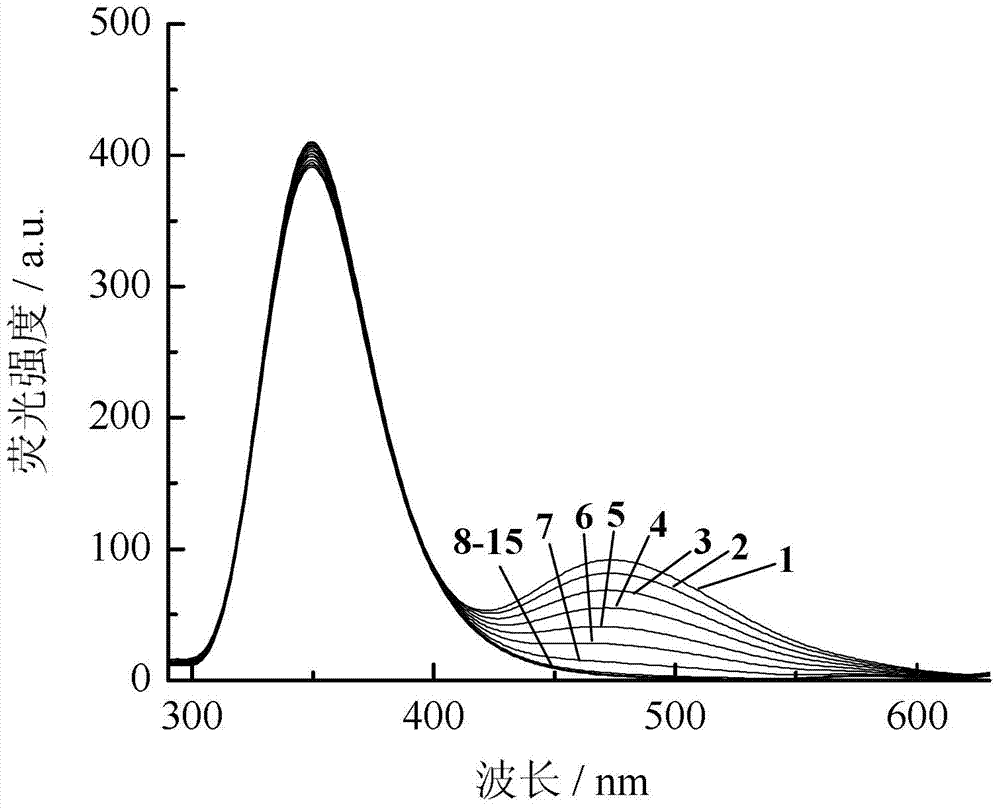
Application of dual-fluorophore ratio fluorescence molecular probe for non-fluorescence resonance energy transfer
A resonance energy transfer, dual fluorophore technology, used in fluorescence/phosphorescence, luminescent materials, material excitation analysis, etc., can solve the problems of low selectivity and sensitivity of metal ions, weak probe fluorescence, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0038] Embodiment 1: In this embodiment, a non-fluorescence resonance energy transfer dual-fluorophore ratio fluorescent molecular probe has the following structural formula:
[0039]
[0040] The FL 1 for where the R 1 Is -H, methyl, 2'-pyridyl or -X, wherein X is a halogen element; said FL 2 for where the R 2 for C 1 ~C 4 Straight-chain alkyl, 2-hydroxyethyl or 2-(2-hydroxyethoxy)ethyl.
[0041] A non-fluorescence resonance energy transfer dual-fluorophore ratio fluorescent molecular probe of this embodiment has the following advantages:
[0042] (1) The fluorescent molecular probe of the present invention can be directly used for the fluorescence ratio detection of metal ions in water, methanol, DMSO, DMF solvent or their mixed solvents, and has higher selectivity and sensitivity;
[0043] (2) The fluorescent molecular probe of the present invention is simple to synthesize, and the raw materials are easy to obtain, and it has high derivatization, and various ...
specific Embodiment approach 2
[0045] Specific embodiment 2: The preparation method of a non-fluorescence resonance energy transfer dual-fluorophore ratio fluorescent molecular probe described in specific embodiment 1 is carried out according to the following steps:
[0046] 1. Add organic compound A, anhydrous piperazine, potassium iodide and N,N-diisopropylethylamine in sequence to acetonitrile and mix evenly, reflux for 5h~7h under the protection of nitrogen, spin the reaction solution dry, and pass through Column chromatographic separation to obtain the intermediate; the molar ratio of the organic compound A described in step one to anhydrous piperazine is 1:2; the anhydrous piperazine described in step one and N,N-diisopropyl ethyl The molar ratio of base amine is 1:2; The molar mass ratio of the anhydrous piperazine described in the step one and potassium iodide is 1mol:(7g~13g); The molar amount of the anhydrous piperazine described in the step one The volume ratio of the amount to acetonitrile is 1m...
specific Embodiment approach 3
[0049] Specific embodiment three: The preparation method of a non-fluorescence resonance energy transfer dual-fluorophore ratio fluorescent molecular probe described in specific embodiment one is carried out according to the following steps:
[0050] 1. Add organic compound A and anhydrous piperazine to dichloromethane in sequence and mix evenly, stir at room temperature and under the protection of nitrogen for 3h to 5h, spin the reaction solution to dryness, and then obtain the intermediate through column chromatography; step 1 The molar ratio of the organic compound A described in and anhydrous piperazine is 1:2; the molar ratio of the organic compound A described in step 1 and the volume ratio of dichloromethane is 1mol:(15L~25L);
[0051] 2. Add the organic compound B, the intermediate obtained in step 1, potassium iodide and N,N-diisopropylethylamine to acetonitrile and mix them uniformly, reflux for 5h-7h under the protection of nitrogen, and spin the reaction solution to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com