A kind of synthesis technique of fexofenadine hydrochloride
A technology of fexofenadine hydrochloride and synthesis process, which is applied in the field of synthesis technology of fexofenadine hydrochloride, can solve the problems of complex process route, high production cost, low total yield, etc., and achieves simplified operation steps and reduced synthesis steps, the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
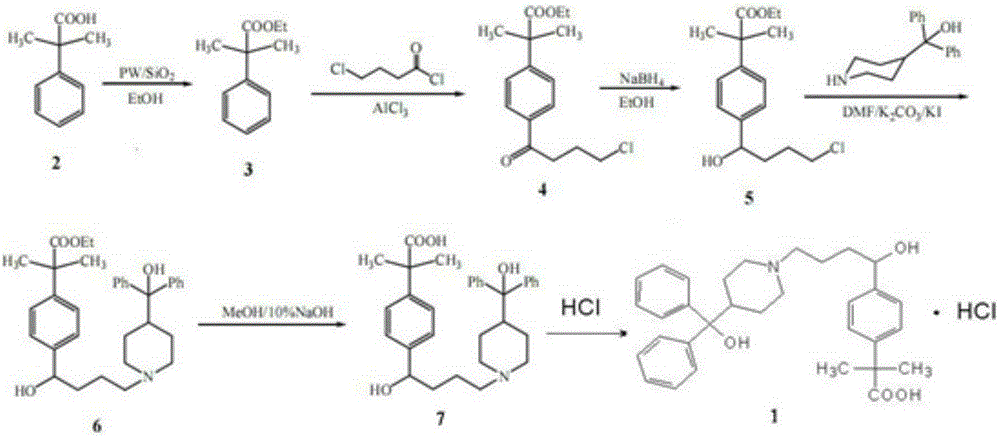
[0017] A kind of synthetic technique of fexofenadine hydrochloride, comprises the following steps:
[0018] (1) Dissolve 0.25mol α, α-dimethylphenylacetic acid in 130ml of absolute ethanol, add 1g of solid-supported heteropolyacid catalyst PW 12 / SiO 2 , heated to reflux to separate water for 3h. Isolate the catalyst (separated by membrane filtration), distill the filtrate to remove ethanol under reduced pressure, dissolve the residue in 250ml of dichloromethane, water (70ml × 2), saturated aqueous sodium bicarbonate (70ml × 2) and water (70ml × 2 ) washed, dried over anhydrous magnesium sulfate and filtered, the filtrate was evaporated under reduced pressure to remove the solvent, the residue continued to be distilled under reduced pressure, and the fraction at 130-133 ° C / 85kPa was collected to obtain a colorless transparent liquid α, α-dimethylphenylacetic acid ethyl Esters 46.1g;
[0019] (2) Dissolve 0.19mol of anhydrous aluminum trichloride in 150ml of dichloromethane...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com