Method for Determining the Response of Acute Myeloid Leukemia to Farnesyltransferase Inhibitor Therapy
A technology for acute myeloid cells and leukemia, applied in the direction of extracellular fluid disease, microbial measurement/test, blood disease, etc., can solve the problem of low response rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0103] Materials and methods
[0104] clinical assessment
[0105] In one example, bone marrow samples were collected from an open-label, multicenter, uncontrolled phase 2 study of tipifarnib in elderly patients with previously untreated high-risk AML To study the efficacy and safety of farnesyltransferase inhibitory therapy.
[0106] Sample collection and processing
[0107] With patient consent, bone marrow samples were collected prior to tipifarnib treatment, and monocytes were subsequently processed on-site. The bone marrow aspirate was diluted with phosphate buffered saline (PBS), and centrifuged with ficoll-diatrizoate (1.077 g / ml). Oxygen-enriched leukemic blood cells were washed twice with PBS, resuspended in PBS containing 10% DMSO, and immediately frozen at -70°C to -80°C. Total RNA was extracted from cell samples using the Trizol kit (Qiagen, Santa Clarita, CA). RNA quality was determined by assessing the presence of ribosomes on an Agilent Bioanalyzer. ...
example 2
[0154] This example describes a modified RT-PCR assay suitable for the application of two gene assays to FTI combination therapy. Taqman assays were designed for the following 3 markers in the analytical assay: RASGRP1 (guanylate exchange factor that activates RAS), APTX (Aprataxin involved in DNA excision repair) and HMBS (for internal reference). HMBS is used as an internal reference in one embodiment, but omitted in other embodiments. The Taqman primer probe sets and their amplicons are listed in the abstract and sequence sections of this application. That is, for APTX-SEQ#3-4 and 2, RASGRP1 primer-probe set SEQ#56 and 1; and HMBS-SEQ#7-9.
[0155] Quantitative RT-PCR assays were developed and optimized using the ABI-7500 platform in a single-tube multiplex format to assess 2-gene ratio performance using FAM-tagged RASGRP1, Gold 540-APTX and Cy5-HMBS. JY Cell RNA and Universal RNA (Stratagene) were used as external normalization.
[0156] RASGRP1:APTX ratio = 2^-"A-B)-(C...
example 3
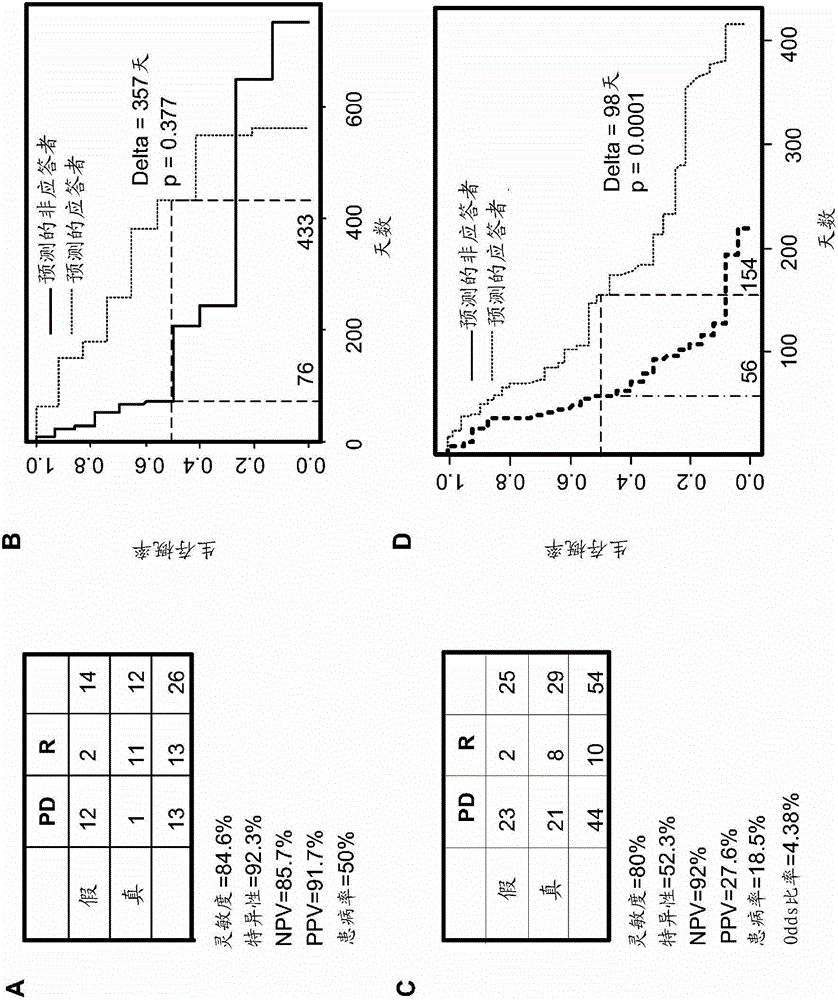
[0190] This example shows that two-gene testing is effective in newly diagnosed AML patients. The 2-gene response prediction assay test was first performed on leukemic cells from a cohort of 84 newly diagnosed AML patients enrolled in a phase 1 dosing study of tipifarnib and etoposide as used in Blood 2008, 111:2589 Describe the RT-PCR protocol. The clinical plan for the phase 1 trial is described in Blood 2009, 113:4841.
[0191] Briefly, 51 unpublished assessed patient samples from the study were analyzed for a preliminary assessment of the performance of the 2-gene assay. Table 11 summarizes the threshold value of 5, RR represents the response rate of CR or PR responders, PPV is the positive predictive value, and NPV is the negative predictive value. Thirteen of 51 patients responded with an RR value of approximately 0.25. Of those above the threshold of 5 on the two-gene assay, half (9 of 18) responded with a PPV of approximately 0.5. Among patients who did not cross t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com