Bone void fillers
A technology of fillers and bone voids, applied in bone diseases, surgical adhesives, prostheses, etc., can solve the problems of expensive and painful acquisition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Intramuscular Implant Evaluation
[0107] To determine whether addition of DiCal would increase bone growth in implanted DBM-containing compositions, various test samples were implanted intramuscularly into athymic rats as described below. Surgery was performed on each rat as follows. Before surgery, rats were anesthetized by intraperitoneal injection of a ketamine / xylazine solution: 70 mg / kg of ketamine and 5 mg / kg of xylazine. General anesthesia was indicated by lack of response to toe pinch. Anesthesia was maintained with isoflurane if necessary.
[0108] The skin over the semitendinosus muscle was shaved (if necessary) using clippers and prepared by scrubbing with chlorhexadine and alcohol. Rats were placed in a lateral position. Using a scalpel or scissors, make a 1 cm incision in the skin along the long bone. Two 2 mm incisions were made in the semitendinosus muscle and blunt dissection was used to prepare the implantation bed.
[0109] Using aseptic techniq...
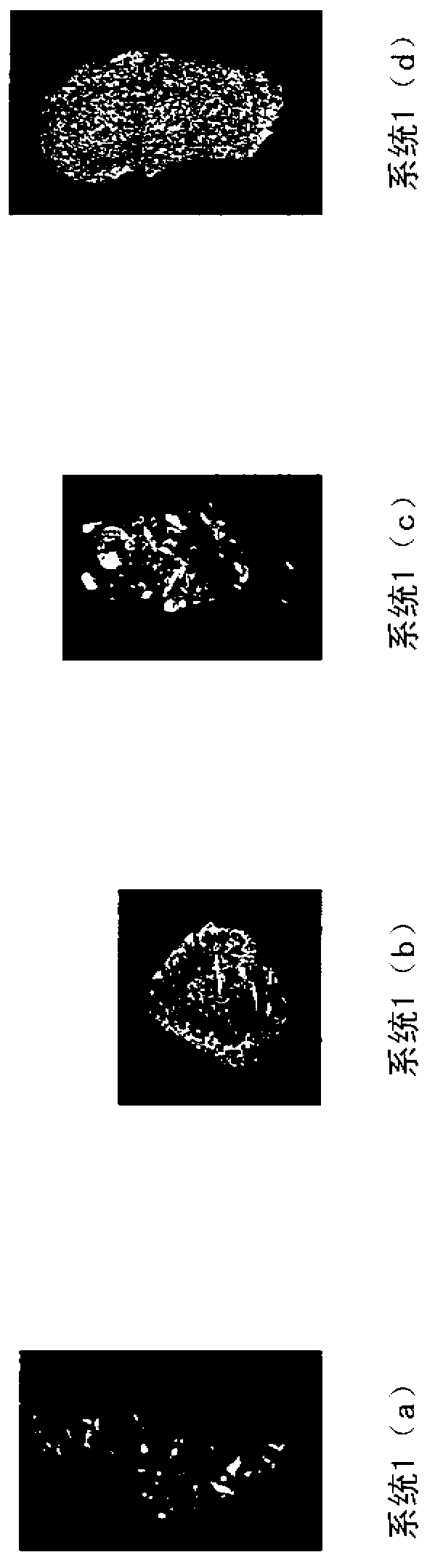
Embodiment 2
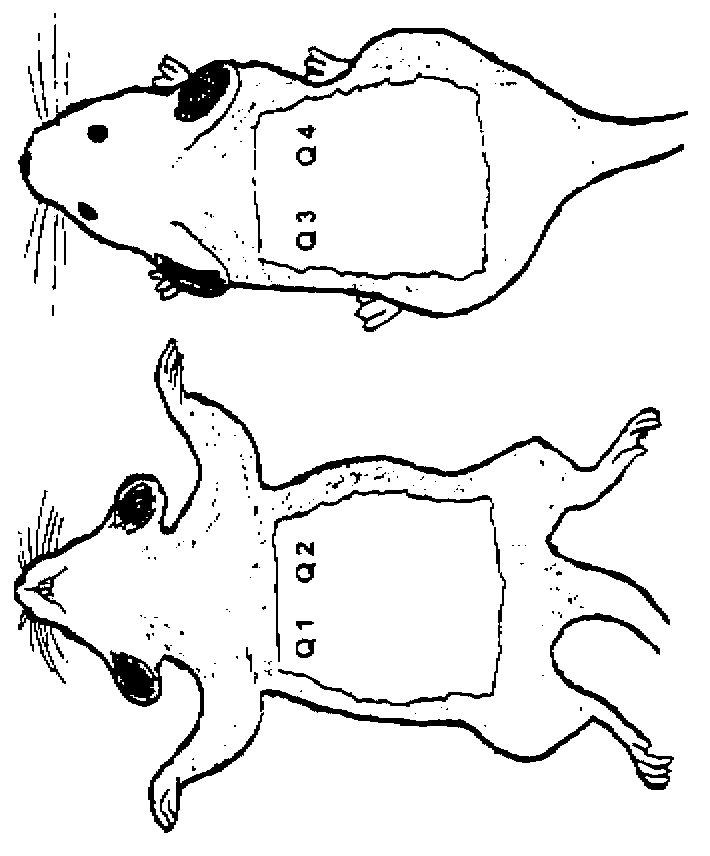
[0116] Subcutaneous Implant Evaluation
[0117] The various systems described below were implanted subcutaneously in athymic rats to evaluate the efficacy of the compositions on bone growth. Male athymic rats were used approximately 7 weeks of age at the start of the study. Surgery was performed on each rat as follows. Before surgery, rats were anesthetized by intraperitoneal injection of a ketamine / xylazine solution: 70 mg / kg of ketamine and 5 mg / kg of xylazine. General anesthesia was indicated by lack of response to toe pinch. Anesthesia was maintained with isoflurane if necessary.
[0118] The ventral and dorsal thorax regions of each rat were swabbed with chlorhexidine and alcohol swabs. Using a scalpel or scissors, make a 1 cm incision in the skin on the right side. This ventral incision is made at the base of the ribcage. After the incision, a pocket is made towards the axilla by blunt dissection under the skin and above the incision. Note the large blood vessels ...
Embodiment 3
[0125] Preparation of Exemplary Putty Compositions
[0126] In this embodiment, gelatin is combined with fibrous collagen, calcium and phosphate sources, and DMB microparticles to form a putty. By whipping 0.18g of collagen, 7.3mL of 30mM hydrochloric acid, 0.21g of calcium hydrogen phosphate (Dical) and 1.6g of demineralized bone microparticles into a slurry, and then freeze-drying the slurry into a 5cm×2cm×0.5cm sponge, The demineralized bone matrix sponge was thus prepared. A 12% w / v gelatin solution was prepared by adding 1.2 g of gelatin to 10 mL of water at about 37°C to about 50°C. Then, the prepared sponge is soaked in water for about 10 minutes to about 30 minutes. After absorbing the water, the sponge is compressed to remove excess water. Then, 1.9 mL of 12% gelatin (at about 37°C to about 50°C) was added to the sponge, spread using a spatula and mixed with the sponge. After 10 minutes, the mixture turned into a putty-like material. The putty material is cohesiv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com