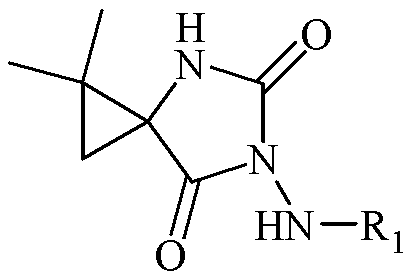
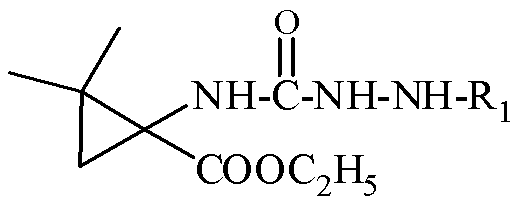N-3-arylamine-5-cyclopropane spirohydantoin and preparation method and application thereof
A spirohydantoin, N-3- technology, applied in pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc., can solve cognitive dysfunction, severe allergic reactions, adverse reactions, etc. problem, to achieve the effect of a simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
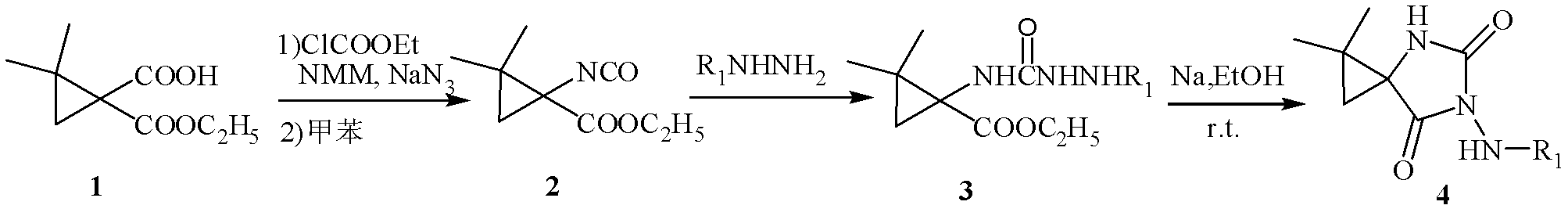
[0032] Embodiment 1: Synthesis of ethyl 1-isocyanate-2,2-dimethylcyclopropanecarboxylate
[0033] Dissolve ethyl 1-carboxy-2,2-dimethylcyclopropanecarboxylate (10mmol) in anhydrous tetrahydrofuran (30mL), cool in an ice-salt bath to about -10°C, and then add ethyl chloroformate (10mmol ) and N-methylpyrrolidone (NMM), immediately produced a white precipitate. At this temperature, after the mixture continued to stir for 20 min, the NaN 3 (10mmol) of 5mL aqueous solution was added to the reaction solution, and stirring was continued for 1h. After the reaction was completed, a small amount of water was added to dissolve the insoluble matter, extracted with ethyl acetate, washed with saturated brine (3×10mL), washed with anhydrous Na 2 SO 4 Let dry overnight. Filter and distill off the solvent under reduced pressure to obtain a light yellow liquid (note: compounds containing azide are explosive and cannot be evaporated to dryness). This crude product is transferred on the sil...
Embodiment 2
[0036] Embodiment 2: Synthesis of ethyl 1-isocyanate-2,2-dimethylcyclopropanecarboxylate
[0037] Dissolve ethyl 1-carboxy-2,2-dimethylcyclopropanecarboxylate (10mmol) in anhydrous tetrahydrofuran (30mL), cool in an ice-salt bath to about -5°C, and then add ethyl chloroformate (30mmol ) and N-methylpyrrolidone (NMM), immediately produced a white precipitate. At this temperature, after the mixture was stirred for 30 min, the NaN 3 (40mmol) of 5mL aqueous solution was added to the reaction solution, and stirring was continued for 1h. After the reaction was completed, a small amount of water was added to dissolve the insoluble matter, extracted with ethyl acetate, washed with saturated brine (3×10mL), washed with anhydrous Na 2 SO 4 Let dry overnight. Filter and distill off the solvent under reduced pressure to obtain a light yellow liquid (note: compounds containing azide are explosive and cannot be evaporated to dryness). This crude product is transferred on the silica gel...
Embodiment 15
[0055] Example 15: Synthesis of 1,1-dimethyl-6-aniline-4,6-diazaspiro[2.4]heptane-5,7-dione
[0056] Dissolve ethyl 2,2-dimethyl-1-phenylhydrazine carboxamide cyclopropanecarboxylate (1mmol) obtained in Example 1 in absolute ethanol (10mL), then add metal Na, react at 5°C, and monitor by TLC , after the reaction was completed, the ethanol was evaporated under reduced pressure, the residue was added a small amount of water, extracted with ethyl acetate (3×20mL), the organic layer was collected, washed with saturated brine, anhydrous Na 2 SO 4 Let dry overnight. After filtration, the solvent was evaporated under reduced pressure and separated by silica gel column chromatography to obtain the compound 1,1-dimethyl-6-aniline-4,6-diazaspiro[2.4]heptane-5,7-dione.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com