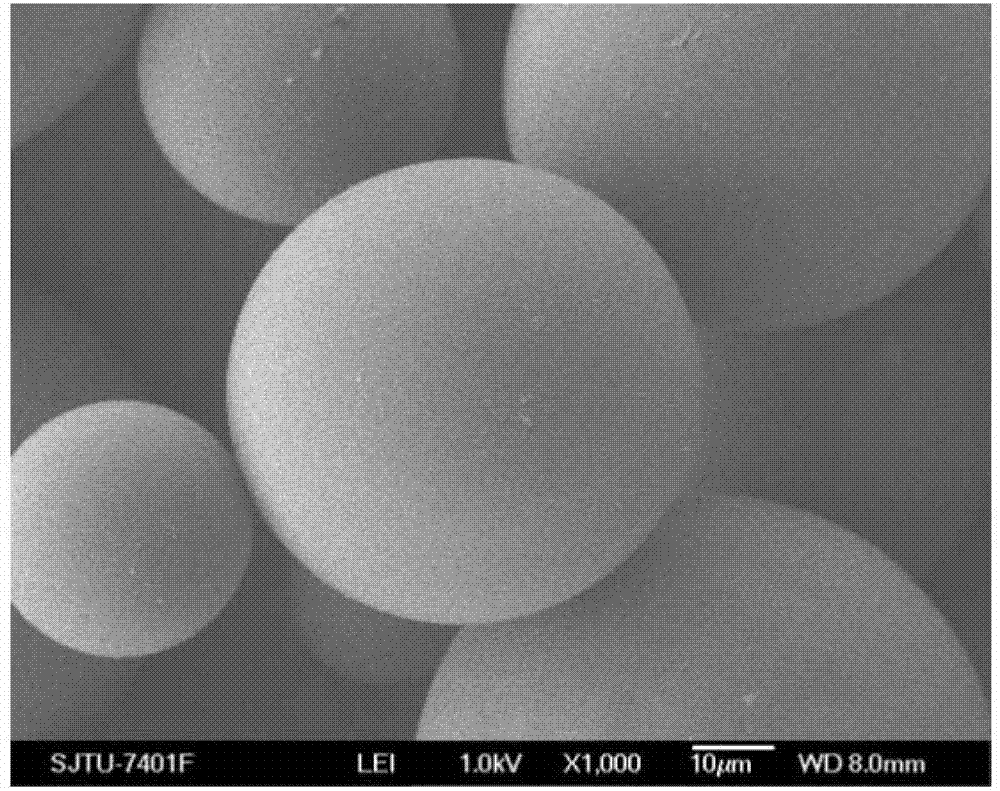
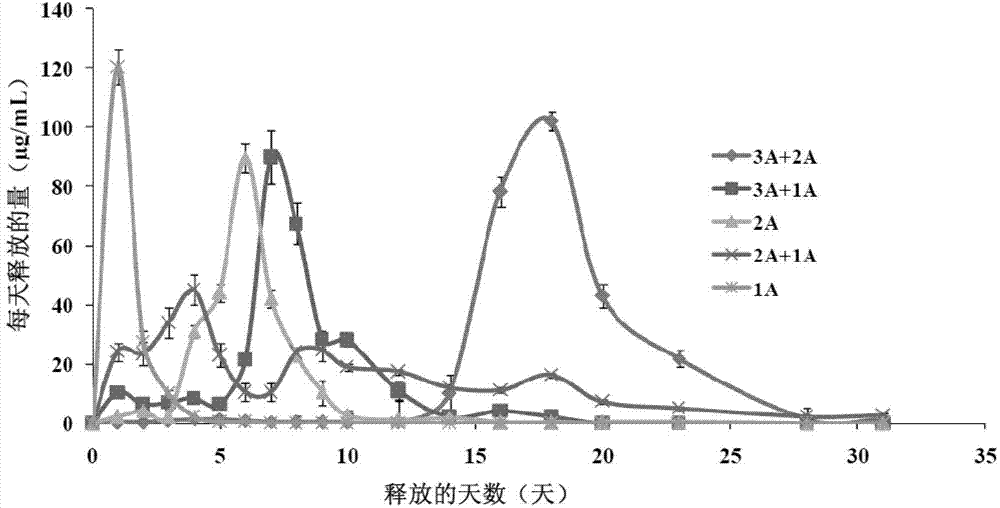
Calcitonin long-acting sustained-release microspheres and preparation method and combination thereof
A technology for sustained-release microspheres and calcitonin, which is applied in the directions of drug combinations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. Small burst release, high encapsulation rate, simple and flexible method of regulating release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of calcitonin long-acting slow-release microspheres includes:
[0033] 1. Preparation of carboxylic acid dextran (Dextran-COOH)
[0034] Weigh a certain amount of dextran sodium carboxylate, select a dialysis bag with a molecular weight of 3500, and dialyze in a hydrochloric acid solution with pH=2 for 24 hours, then replace with a new solution, continue to dialyze with a hydrochloric acid solution with pH=2 for 24 hours, and then use pure water Dialyze for 24 hours, transfer to a small beaker and pre-freeze in a -20°C refrigerator for 12 hours, and finally freeze-dry in a freeze dryer before use.
[0035] Preparation of calcitonin microspheres by 2W / O / W method
[0036] This step includes the preparation of five kinds of calcitonin microspheres, wherein the type and content of PLGA are different, see Table 1, the preparation process of these five kinds of calcitonin microspheres is the same, including the following steps:
[0037] Weigh an appropriate ...
Embodiment 2
[0064] The preparation of calcitonin long-acting slow-release microspheres includes:
[0065] 1. Dextran sulfate (Dextran-SO 3 OH) and preparing granules from calcitonin and dextran sulfate
[0066] Weigh 50g of dextran sodium sulfate, select a dialysis bag with a molecular weight of 3500, and dialyze in a hydrochloric acid solution with pH=2 for 24 hours, then replace with a new solution, continue to dialyze with a hydrochloric acid solution with pH=2 for 24 hours, and then dialyze with pure water for 24 hours , transferred to a beaker and added with a mass concentration of 5% calcitonin solution and 500g of PEG (molecular weight: 8000Da) with a mass concentration of 10%, pre-freezing in a refrigerator at -20°C for 12 hours, and finally putting it into a lyophilizer for lyophilization. PEG was removed with dichloromethane for later use.
[0067] Preparation of calcitonin microspheres by 2S / O / W method
[0068] This step includes the preparation of five kinds of calcitonin m...
Embodiment 3
[0078] 1. Dextran sulfate (Dextran-SO 3 OH) preparation
[0079] Weigh a certain amount of dextran sodium sulfate, select a dialysis bag with a molecular weight of 3500, and dialyze in a hydrochloric acid solution of pH=2 for 24 hours, then replace with a new solution, continue to dialyze with a hydrochloric acid solution of pH=2 for 24 hours, and then dialyze with pure water 24h, transferred to a small beaker and pre-frozen in a -20°C refrigerator for 12h, and finally placed in a lyophilizer for freeze-drying before use.
[0080] Preparation of Calcitonin Long-acting Sustained-release Microspheres by 2W / O / W Method
[0081] This step includes the preparation of five kinds of calcitonin long-acting sustained-release microspheres, wherein the type and content of PLGA are different, see Table 5, the preparation process of these five kinds of calcitonin long-acting sustained-release microspheres is the same, including the following steps:
[0082] Weigh an appropriate amount of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com