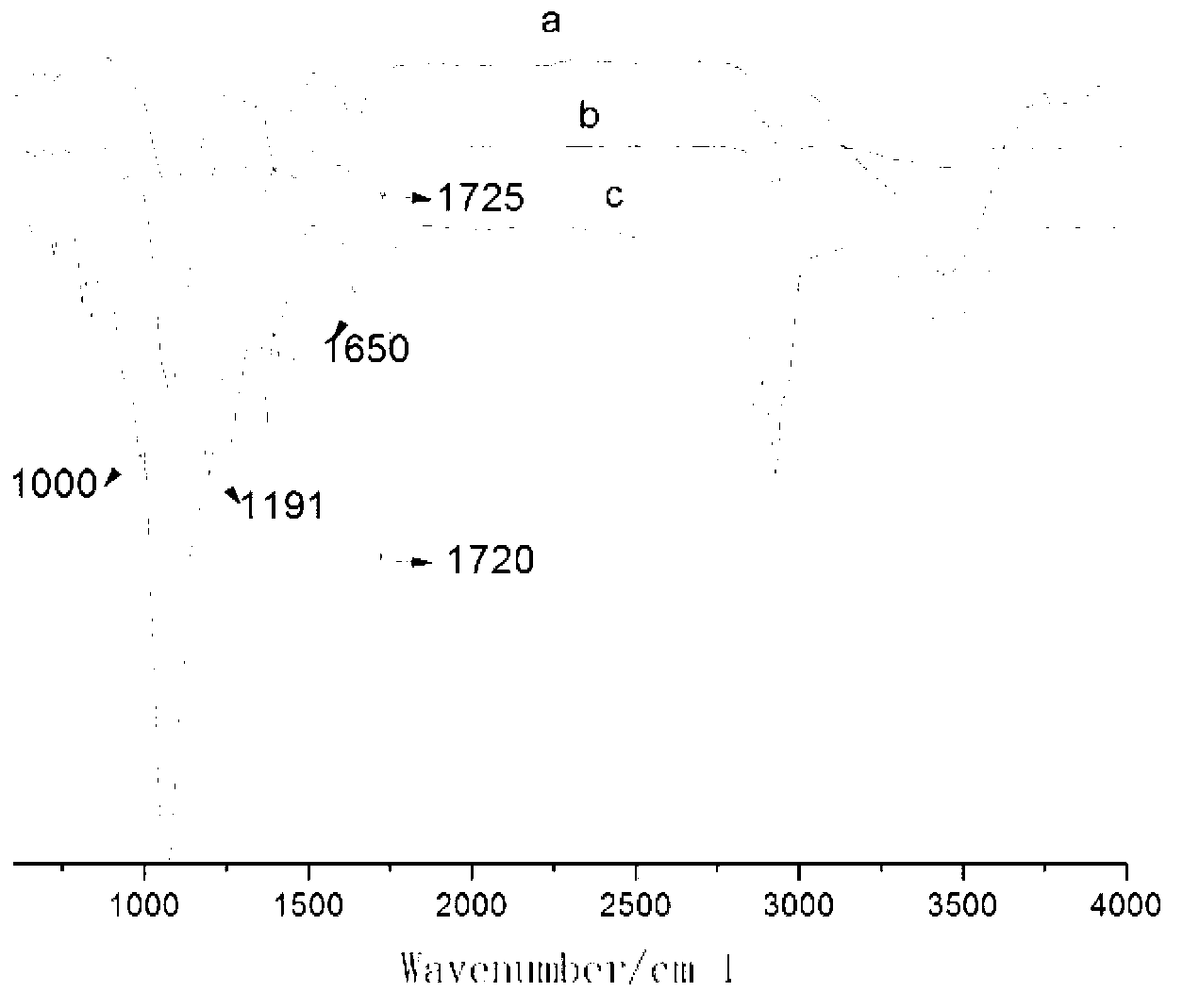Surface-heparinized cellulose ester liquid crystal material and preparation method and application thereof
A technology of liquid crystal materials and cellulose, applied in liquid crystal materials, chemical instruments and methods, etc., can solve the problems of surface liquid crystal state structure change, deformation, insufficient stability of small molecule liquid crystal domains, etc., to reduce interfacial tension, mature technology, high efficiency The effect of anticoagulant properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
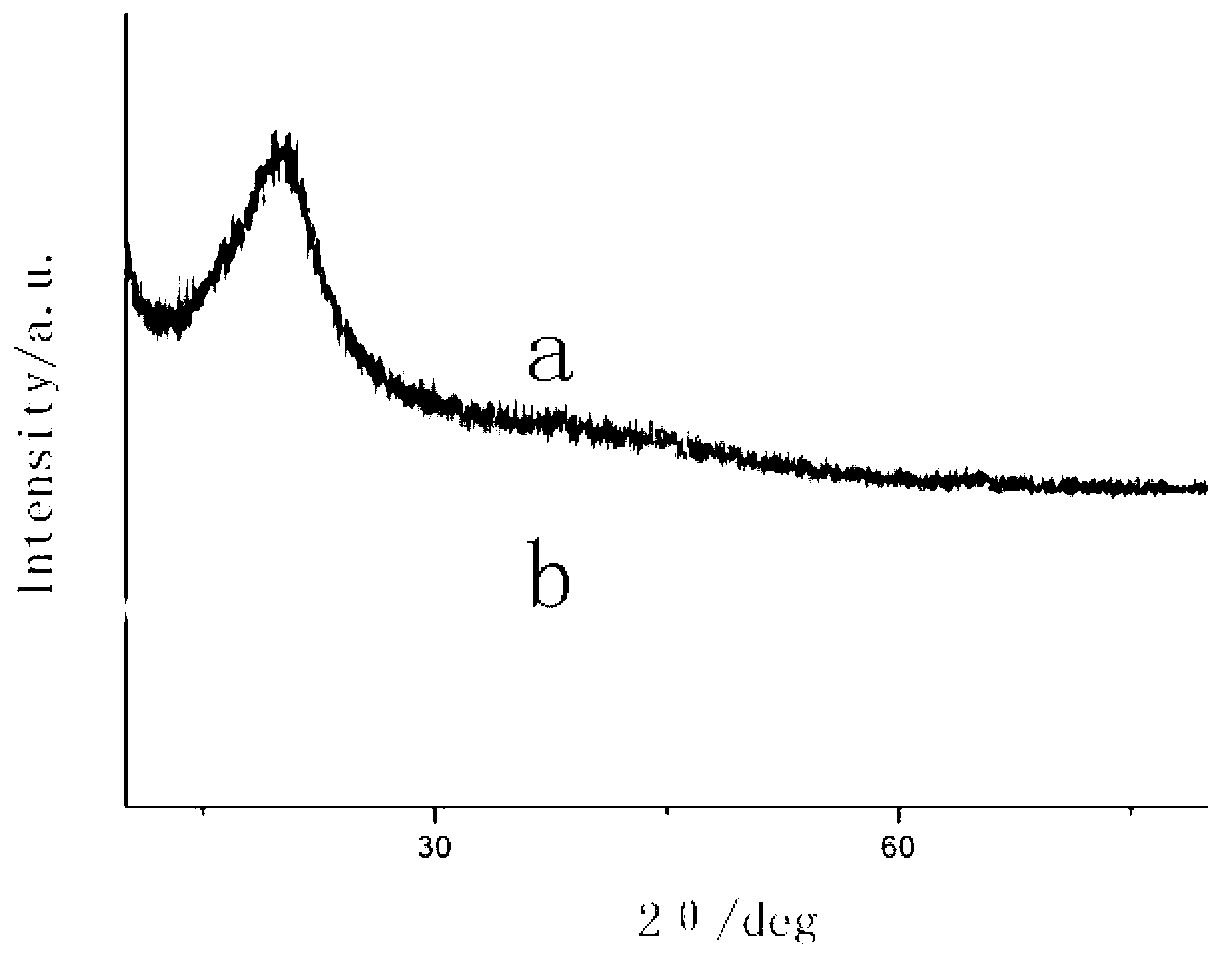
Image
Examples
Embodiment 1
[0035] (1) Preparation of acryloyl-octanoyl-hydroxypropyl cellulose ester liquid crystal: 2 g of hydroxypropyl cellulose (M W =100,000, purchased from Aldrich Company) and dissolved in acetone solution; after complete dissolution, 1 mL of acryloyl chloride was added dropwise to it, and the reaction was carried out at 55 °C for 4 h. After the reaction, 200 mL of deionized water was added to the mixed solution to precipitate the product, and stirred for 5 h Then, let stand for 15 minutes, remove the aqueous solution, dissolve the precipitated product in acetone, add 200 mL of deionized water to precipitate the precipitated product, stir for 5 h, let stand for 15 minutes, remove the aqueous solution, dissolve the precipitate in acetone, repeat the above After adding deionized water, stirring, standing, removing water, and dissolving the precipitate in acetone for 5 times, at 25 °C, dialyzed in deionized water for 48 h to remove residual acryloyl chloride, and dried; then the obtai...
Embodiment 2
[0051] (1) Preparation of acrylated-octanoylated-hydroxypropyl cellulose ester liquid crystal: 1 g of hydroxypropyl cellulose (M W = 200,000, purchased from Aldrich Company) and dissolved in acetone solution; after complete dissolution, 0.5 mL of acryloyl chloride was added dropwise, and the reaction was carried out at 65 °C for 3 h. After the reaction, 200 mL of deionized water was added to the mixed solution to precipitate the precipitated product. After stirring for 5 hours, let stand for 15 minutes, remove the aqueous solution, dissolve the precipitated product in acetone, add 200 mL of deionized water to separate out the precipitated product, stir for 5 hours, let stand for 15 minutes, remove the aqueous solution, and dissolve the precipitate in acetone, After repeating the above process of adding deionized water, stirring, standing, removing water, and dissolving the precipitate in acetone for 5 times, at 25 °C, dialyzed in ethanol for 8 h to remove residual acryloyl chlo...
Embodiment 3
[0054] Preparation of acrylated-octanoylated-hydroxypropyl cellulose ester liquid crystal: 20 g of hydroxypropyl cellulose (M W = 80,000, purchased from Aldrich Company) and dissolved in acetone solution; after complete dissolution, 10 mL of acryloyl chloride was added dropwise to it, and the reaction was carried out at 45 °C for 6 h. After the reaction, 200 mL of deionized water was added to the mixed solution to precipitate the product, and stirred for 5 h Then, let stand for 15 minutes, remove the aqueous solution, dissolve the precipitated product in acetone, then add 200 mL of deionized water to precipitate the precipitated product, stir for 5 h, let stand for 15 minutes, remove the aqueous solution, dissolve the precipitate in acetone, repeat The above process of adding deionized water, stirring, standing, removing water, and dissolving the precipitate in acetone for 3 times, at 25 ° C, dialyzed in tetrahydrofuran for 8 h to remove residual acryloyl chloride, and dried; t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com