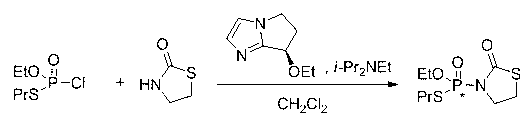
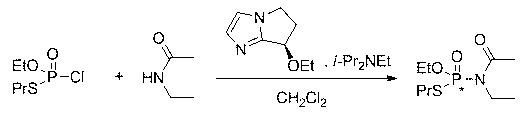
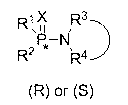Chiral phosphamide compound and preparation method thereof
A technology of phosphoramides and compounds, applied in the field of bioactive drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: 7-Hydroxy-6,7-dihydro-5 H - Preparation of pyrrole [1,2-α] imidazole
[0030] Add imidazole (50.0 g, 0.734 mol), glacial acetic acid (3.0 mL, 0.051 mol, 0.07 eq), solvent 1,4-dioxane (500 mL) into a 1 L three-necked flask, and stir to dissolve at room temperature. Then pour freshly steamed acrolein (63.0 mL, 0.943 mol, 1.3 eq) in one go, and reflux for 48 h. Then the volatile solvent was evaporated under reduced pressure, and the obtained solid crude product was separated by column chromatography (EtOAc / MeOH = 3 / 1) to obtain 73.8 g of light yellow solid compound with a yield of 81%.
[0031] 1 H NMR (400 MHz, CDCl 3 ): δ 7.07 (d, J = 1.2 Hz, 1H), 6.85 (d, J = 1.2 Hz, 1H), 5.23 (dd, J = 3.2 Hz, 7.2 Hz, 1H), 4.24-4.16 (m, 1H) , 3.98-3.98 (m, 1H), 3.00-2.88 (m, 1H), 2.64-2.54 (m, 1H).
[0032] 13 C NMR (100 MHz, CDCl 3 ): δ 156.4, 132.5, 114.3, 63.6, 43.2, 36.3.
Embodiment 2
[0033] Example 2: (+)-7-Hydroxy-6,7-dihydro-5 H -pyrrole[1,2-α]imidazole and (-)-7-hydroxy-6,7-dihydro-5 H - Preparation of pyrrole [1,2-α] imidazole
[0034] In a dry 250 mL two-necked bottle, 7-hydroxy-6,7-dihydro-5 H -Pyrrole[1,2-α]imidazole (14.5 g, 0.11 mol) was dissolved in 100 ml of methanol, and (+)-tartaric acid (17.5 g, 0.11 mol, 1.0 eq) in methanol solution 100 mL was added under reflux with stirring, and refluxed for 2 h. After cooling to room temperature, a light yellow solid was precipitated (the solid was basified with an appropriate amount of NaOH to free 7-hydroxyl-6,7-dihydro-5 H -pyrrole[1,2-α]imidazole with an ee value of about 30%). The above-mentioned pale yellow solid was recrystallized several times with 200 mL of methanol and a small amount of water to obtain a solid, which was basified with NaOH, extracted with dichloromethane, and 0.6 g of a white powdery solid was obtained after evaporating the solvent, with a yield of 4%, ee= 99.4% (Daicel CHI...
Embodiment 3
[0035] Example 3: (+)-7-methoxy-6,7-dihydro-5 H - Preparation of pyrrole [1,2-α] imidazole
[0036] Add (+)-7-hydroxy-6,7-dihydro-5 to a dry 25 mL two-necked bottle H -Pyrrole [1,2-α] (50.5 mg, 0.4 mmol), inject dry THF (5 mL), stir to dissolve, add NaH (16.1 mg, 0.4 mmol, 1.0 eq) under nitrogen protection, stir at room temperature for 2 h , then slowly drop into MeI (25.0 μL, 0.4 mmol, 1.0 eq), and stir at room temperature for 15 h. After evaporating the volatile solvent, extract with dichloromethane (15 ml × 3), the combined dichloromethane phase was dried over sodium sulfate, dichloromethane was evaporated under reduced pressure, and silica gel column chromatography (EtOAc / MeOH = 10 / 1 , Rf = 0.44) to obtain 23.8 mg of light yellow oily liquid with a yield of 42%.
[0037] 1 H NMR (400 MHz, CDCl 3 ): δ 7.13 (s, 1 H), 6.92 (d, J = 1.2 Hz, 1H), 4.63 (dd, J = 7.2 Hz, 2.0 Hz, 1H), 4.18-4.10 (m, 1 H), 3.52 (s, 3H), 2.86-2.80 (m, 1H), 2.60-2.46 (m, 1H).
[0038] 13 C N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com