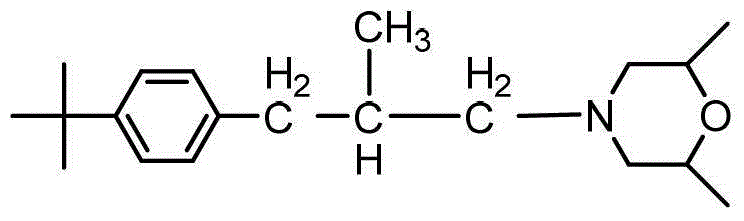
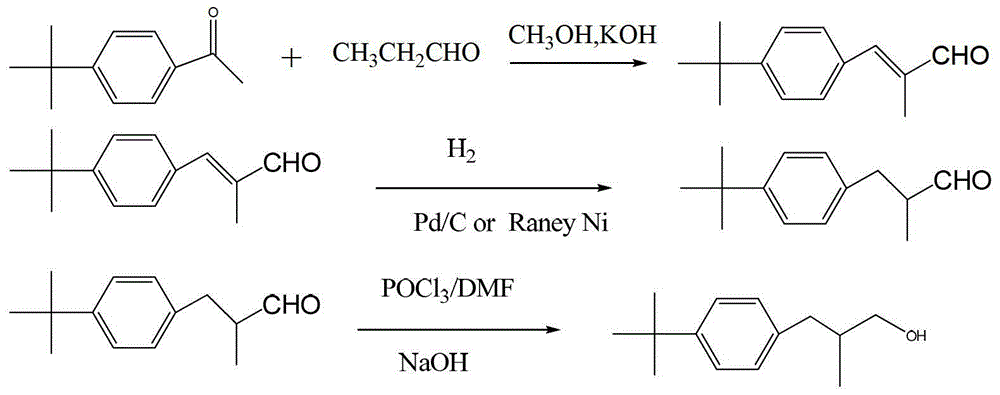
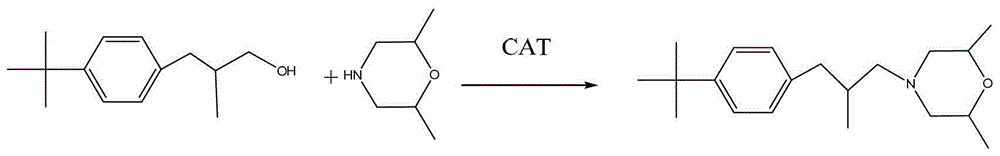
Synthesis method of fenpropimorph
A synthesis method and technology of fenpropimorph are applied in the field of synthesis of fenpropimorph, can solve problems such as no industrial production value, and achieve the effects of low cost, simple equipment and high content of finished products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 206g of p-tert-butyl-β-methylphenylpropanol and 120g of methanesulfonyl chloride into a 500ml round-bottomed flask, cool down to 0°C, add 111g of triethylamine dropwise, and keep the temperature for 1 hour after the dropwise addition is complete. Sampling and analysis until qualified. Add 200g of water after the heat preservation finishes, let stand and separate. The lower layer is the intermediate sulfonate after reaction. Weighed 282.6g of the intermediate, and the analytical content (GC normalization method) was 99.0%. The yield is 99.5%.
[0029] Put the sulfonate ester synthesis product of the first step into a 1000ml four-necked flask, add 345g of 2,6-dimethylmorpholine, heat up and reflux until the temperature rises to 140°C, keep it warm for 4 hours, and then neutralize it with 30% NaOH aqueous solution To PH = 14, static separation, the upper oil layer vacuum distillation, after cutting off the former fraction to collect the finished fenpropimorph. Weig...
Embodiment 2
[0031] Add 103g of p-tert-butyl-β-methylphenylpropanol and 60g of methanesulfonyl chloride into a 500ml round bottom flask, cool down to 0°C, add 56g of triethylamine dropwise, and keep the temperature for 1 hour after the dropwise addition is complete. Sampling and analysis until qualified. Add 100g of water after the heat preservation finishes, and let stand to stratify. The lower layer is the intermediate sulfonate after reaction. 141.4 g of the intermediate was obtained by weighing, and the analytical content (GC normalization method) was 98.9%. The yield is 99.5%.
[0032] Put the sulfonate ester synthesis product of the first step into a 500ml four-neck flask, add 173g of 2,6-dimethylmorpholine, heat up and reflux until the temperature rises to 140°C, keep it warm for 4 hours, and then neutralize it with 30% NaOH aqueous solution To PH = 14, static separation, the upper oil layer vacuum distillation, after cutting off the former fraction to collect the finished fenpro...
Embodiment 3
[0034] Add 412g of p-tert-butyl-β-methylphenylpropanol and 240g of methanesulfonyl chloride into a 1000ml round bottom flask, cool down to 0°C, add 222g of triethylamine dropwise, and keep the temperature for 1 hour after the dropwise addition. Sampling and analysis until qualified. Add 400g of water after the heat preservation finishes, and let stand to stratify. The lower layer is the intermediate sulfonate after reaction. 567.4g of the intermediate was obtained by weighing, and the analytical content (GC normalization method) was 98.5%. The yield is 99.4%.
[0035] Put the sulfonate ester synthesis product of the first step into a 2000ml four-necked flask, add 690g of 2,6-dimethylmorpholine, raise the temperature and reflux until the temperature rises to 140°C, keep it warm for 4 hours, and then neutralize it with 30% NaOH aqueous solution To PH = 14, static separation, the upper oil layer vacuum distillation, after cutting off the former fraction to collect the finished...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com