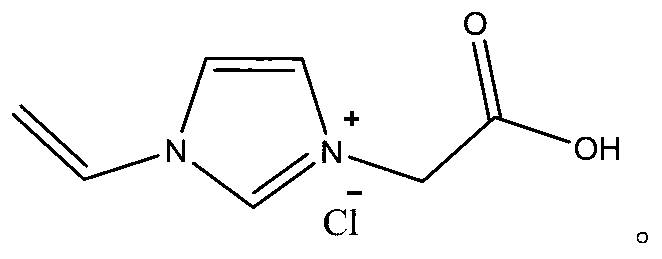
Chlorinated 1-vinyl-3-carboxymethyl imidazole polymerizable acidic ionic liquid and synthetic method thereof
A technology of acidic ionic liquid and carboxymethylimidazole, which is applied in the field of functional materials and its preparation, can solve the problems of low reaction yield, low solubility, and difficult removal, etc., and achieve the effect of improving yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Dissolve 3.00g (31.75mmol) of 2-chloroacetic acid in 9g of ethyl acetate, stir well at 50°C for 20min to obtain a clear ethyl acetate solution containing 2-chloroacetic acid, and add 0.03g of Polymerization inhibitor phenol, stir to dissolve, then add 2.99g (31.75mmol) of 1-vinylimidazole to it, react at 45°C for 48 hours, a white precipitate is obtained, wash with ethyl acetate 3 times, each time 30mL, filter , put the obtained white solid into a vacuum drying oven at 50° C. for 3 h under vacuum to obtain 4.67 g of 1-vinyl-3-carboxymethylimidazole chloride with a yield of 78%.
Embodiment 2
[0022] Dissolve 4.50g (47.62mmol) of 2-chloroacetic acid in 36g of ethyl acetate, stir well at 50°C for 20min to obtain a clear ethyl acetate solution containing 2-chloroacetic acid, and add 0.03g of Polymerization inhibitor phenol, stirred and dissolved, then added 2.99g (31.75mmol) of 1-vinylimidazole to it, reacted at 48°C for 24 hours, a white precipitate was obtained, washed 3 times with ethyl acetate, 30mL each time, filtered , put the obtained white solid into a vacuum drying oven at 50° C. for 3 h under vacuum to obtain 4.79 g of 1-vinyl-3-carboxymethylimidazole chloride with a yield of 80%.
Embodiment 3
[0024] Dissolve 6.00g (63.50mmol) of 2-chloroacetic acid in 30g of ethyl acetate, stir well at 50°C for 20min to obtain a clear ethyl acetate solution containing 2-chloroacetic acid, and add 0.03g of Polymerization inhibitor phenol, stir to dissolve, then add 2.99g (31.75mmol) of 1-vinylimidazole to it, react at 50°C for 18 hours, a white precipitate is obtained, wash with ethyl acetate 3 times, each time 30mL, filter , put the obtained white solid into a vacuum drying oven at 50° C. for 3 h under vacuum to obtain 4.93 g of 1-vinyl-3-carboxymethylimidazole chloride, with a yield of 82.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com