Method for preparing carbon-coated lithium iron phosphate from basic lithium iron phosphate
A technology for carbon-coated lithium iron phosphate and lithium iron phosphate is applied in the field of preparation of lithium iron phosphate and basic lithium iron phosphate to prepare carbon-coated lithium iron phosphate, which can solve problems such as undisclosed specific methods and achieve stable product performance. , The process is simple and the controllability is good.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
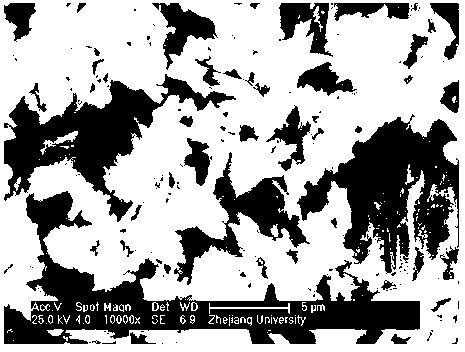
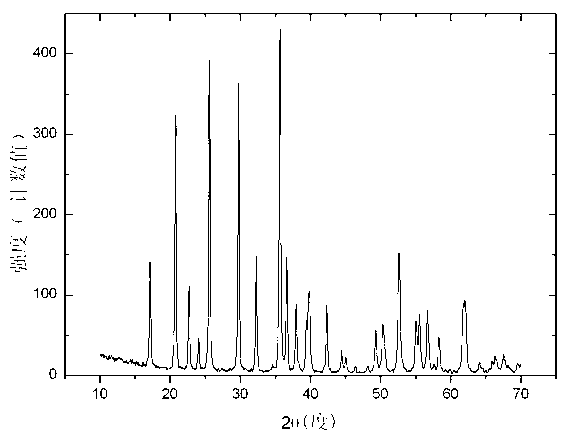
Image
Examples
Embodiment 1
[0059] 1mol iron phosphate FePO 4 , 1mol lithium hydroxide LiOH and 4L deionized water were put into a ball mill and milled for 1 hour to obtain a mixture; then the mixture was added to the reaction kettle, reacted at 120°C for 32 hours, filtered, and dried to obtain the chemical formula LiFePO 4 (OH) basic lithium iron phosphate.
Embodiment 2
[0061] 1mol iron phosphate FePO 4 , 1.02mol lithium hydroxide LiOH and 4.5L deionized water were put into a ball mill and milled for 2 hours to obtain a mixture; then the mixture was added to the reactor, reacted at 200°C for 20 hours, filtered, and dried to obtain the chemical formula: LiFePO 4 (OH) basic lithium iron phosphate.
Embodiment 3
[0063] 1mol iron phosphate FePO 4 , 1.05mol lithium hydroxide LiOH and 5L deionized water were put into a ball mill and milled for 3 hours to obtain a mixture; then the mixture was added to the reactor, reacted at 250°C for 6 hours, filtered, and dried to obtain the chemical formula LiFePO 4 (OH) basic lithium iron phosphate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com