Method and device for isolating cells from heterogeneous solution using microfluidic trapping vortices
A microfluidic device and a technology for separating cells, which can be used in measuring devices, fluid controllers, and laboratory containers, and can solve problems such as useless capture of cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1 - Enrichment of rare cancer cells from blood
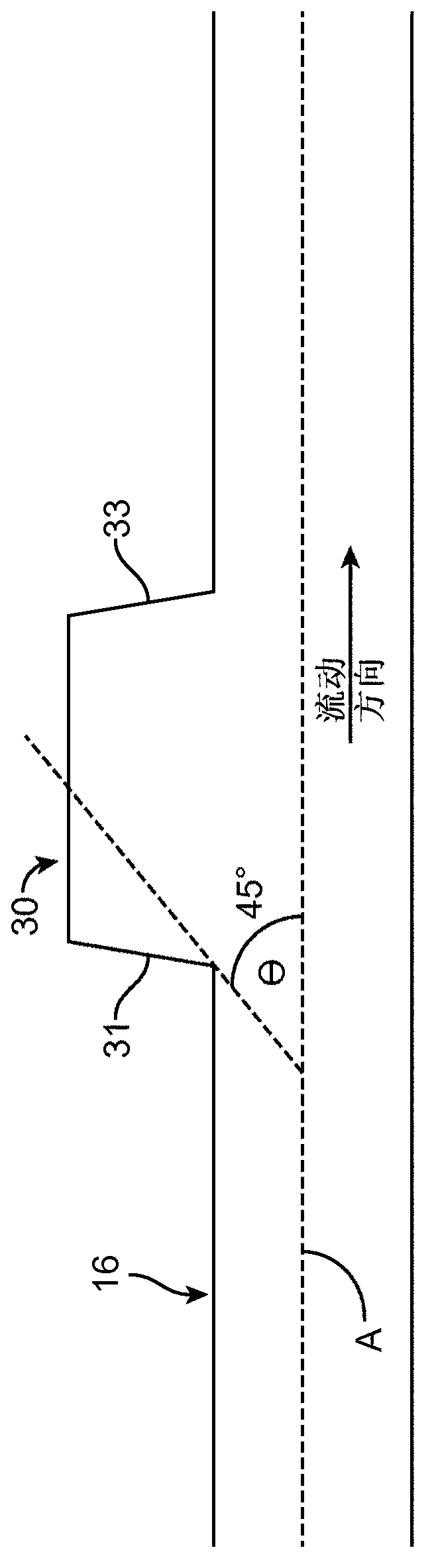
[0060] image 3 The microfluidic device 10 was adapted to isolate and concentrate cancer cells (20 microns in diameter) from normal human blood cells (2 to 15 microns in diameter) to demonstrate size-based enrichment and concentration in a high-throughput manner. utility. For clinical diagnosis, the enrichment and concentration of cancer cells from blood is of particular importance, as circulating tumor cells (CTCs) can provide real-time information on patient pathology and monitoring of cancer treatment. Isolation of live CTCs from blood in a rapid, efficient and label-free manner remains a major technical challenge: CTCs are rare events with rates as low as one cell per billion blood cells. While current strategies focus on the investigation of CTCs for diagnosis, there is a significant need to collect larger sample volumes of live CTCs for research purposes. This requires higher throughput processing of la...
Embodiment 2
[0069] Example 2 - Cell labeling and solution replacement
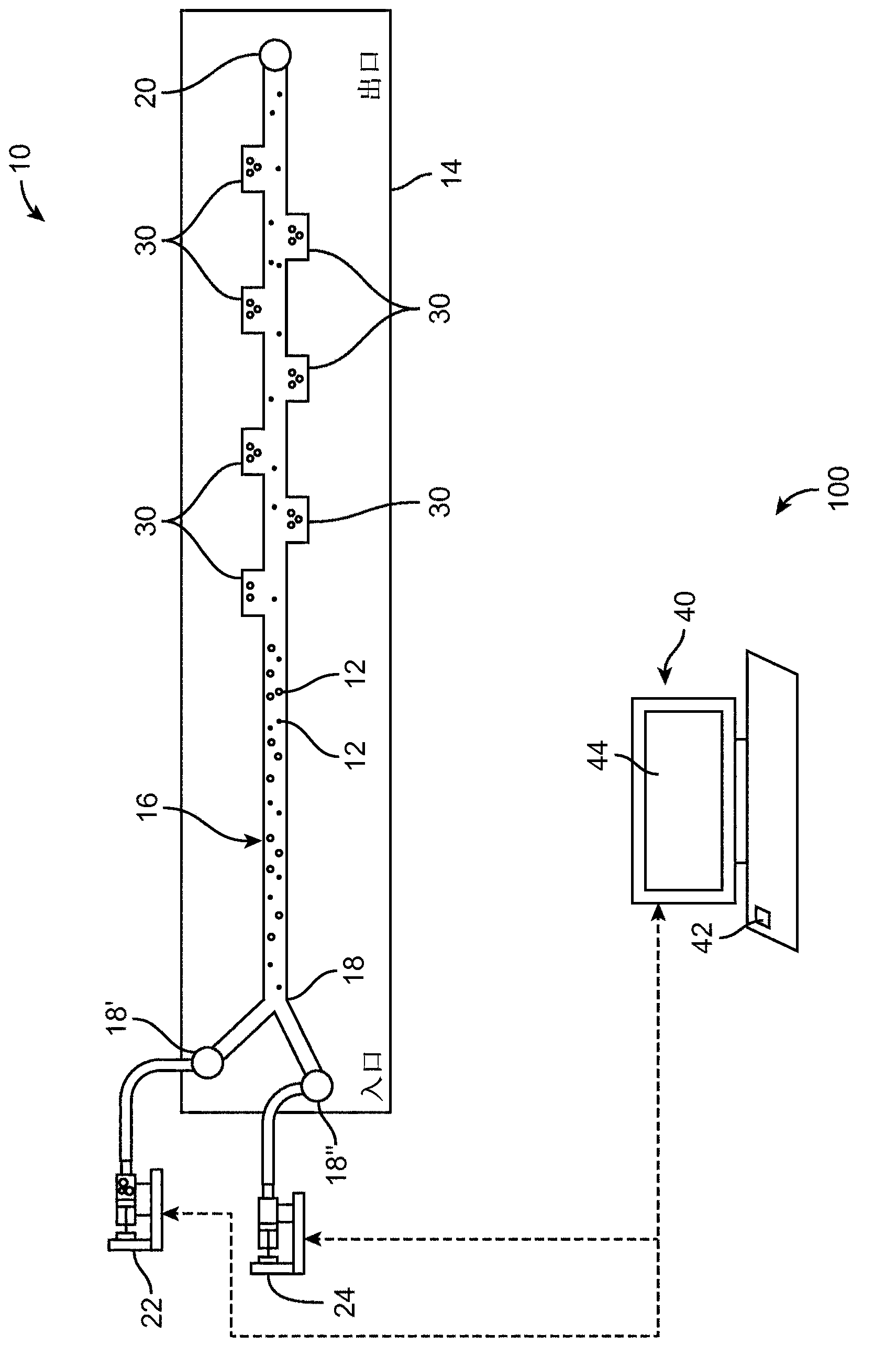
[0070] The microfluidic device 10 is also used to efficiently label cells with specific molecular markers. In conventional centrifugation, a sample of cells is labeled with a specific marker through a series of labeling and washing steps. This involves incubating the cells with the labeling reagent in a centrifuge tube, concentrating the cells to a pellet in a benchtop centrifuge, removing the supernatant containing unbound labeling reagent by manual pipetting, and manually resuspending the cells in new medium. These manipulations were performed within the microfluidic device 10 by trapping the cells in a fluid vortex and then exposing the trapped coiled cells to a labeling reagent followed by a PBS wash. Labeled cells are then released into collection vials in small volumes by reducing the flow rate.
[0071] Figure 6A-6D respectively shows the interception ( Figure 6A ), first solution replacement ( Figure...
Embodiment 3
[0074] Example 3 - Sequential operation: enrichment of rare cells followed by labeling
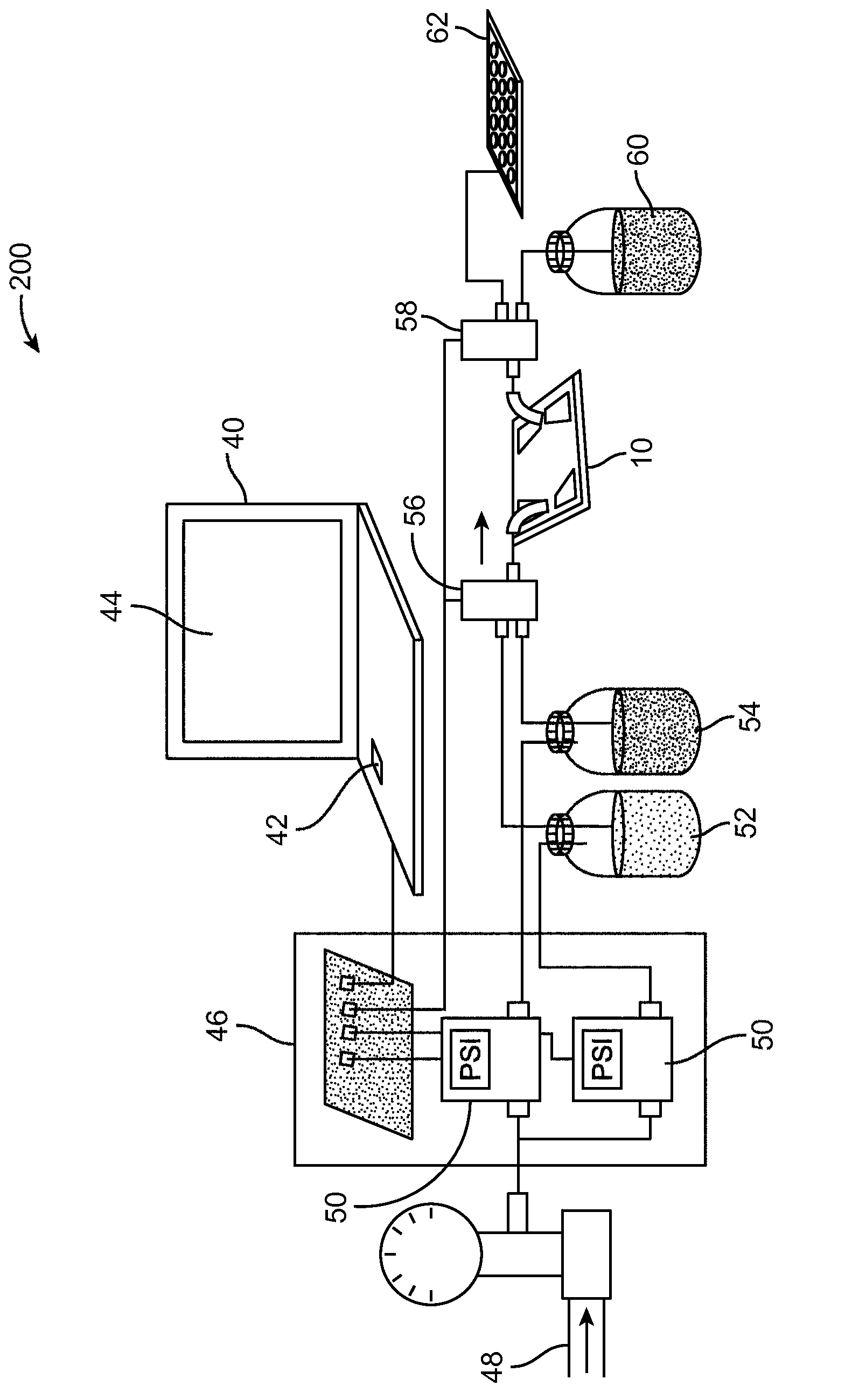
[0075] use Figure 7 The microfluidic device 10 shown successfully performed the multiple sequential sample preparation steps enabled by the centrifuge (eg, entrapped fluorescent solution exchange, reaction, and wash). In this embodiment, the microfluidic device 10 includes three inlets 18', 18'', and 18'''. One inlet 18' is connected to a syringe pump 22 for delivering the cell sample. The second syringe pump 24 is used to deliver the fluorescent agent. The third syringe pump 26 is used to deliver washing solution (PBS). Size-based retention of cancer cells from blood, serial fluorescent labeling, and analysis of released cells were performed in Figure 8A-Figure 8D The order of interception, first solution replacement, reaction, and second solution replacement is shown. Cells were then washed Figure 9 , which shows fluorescent images of cell clusters that were sequentially tr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com