Polymerase chain reaction (PCR) authentication primer and method for distinguishing bradysia odoriphaga larva from bradysia difformis larva
A larval, one-to-one technique for use in agrobiology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Extraction of genomic DNA of chive maggots and bacterial maggots
[0037] Place the single-headed chive maggots and bacterial maggots in 0.2ml centrifuge tubes containing 60 μl of alkaline lysate, respectively: 50 mmol L -1 Tris-HCl (pH8.0), 20mmol·L -1 NaCl, 1mmol L -1 EDTA (ethylenediaminetetraacetic acid) and 1% SDS (sodium dodecyl sulfate) are fully ground and homogenized with a sealed gun head, placed in a water bath at 65°C for 15 minutes, and then placed in a water bath at 95°C for 10 minutes to obtain Chive maggot and bacterial maggot genome DNA solution.
[0038] (2) PCR amplification of COI genes of chive maggots and bacterial maggots
[0039] Carrying out PCR amplification with the chive maggot genome DNA solution and the bacteria maggot genome DNA solution as templates respectively to obtain PCR amplification products;
[0040] The PCR amplification system is:
[0041] Chive maggot genomic DNA solution: 3μl; 20μM primer: 0.5μl; 5U / μl Taq enzyme: 0.5...
Embodiment 2
[0049] A method for distinguishing leek maggots and bacterial maggots, the steps are as follows:
[0050] (1) Place the individual leek maggots and bacterial maggots collected in Jinan City, Shandong Province into 0.2ml centrifuge tubes containing 60μl of alkali lysis solution, the alkali lysis solution is: 50mmol L -1 Tris-HCl (pH8.0), 20mmol·L -1 NaCl, 1mmol L -1 EDTA, 1% SDS, fully grind the homogenate with a sealed pipette tip, put it in a water bath at 65°C for 15 minutes, and then put it in a water bath at 95°C for 10 minutes to obtain a genomic DNA solution;
[0051] (2) Using the genomic DNA prepared in step (1) as a template, performing PCR amplification on the mitochondrial COI gene in the genomic DNA to obtain a PCR amplification product;
[0052] The PCR amplification system is:
[0053] Genomic DNA solution 2μl, 20μM primer 0.5μl, 5U / μl Taq enzyme 0.25μl, 10×Taq Buffer 2.5μl, 10mM dNTP 0.5μl, ddH 2 0 to 25 μl;
[0054] The primer sequences are as follows:
...
Embodiment 3
[0063] The method for identifying leek maggots and bacterial maggots as described in Example 2, the difference is that the leek maggots and bacterial maggots were collected in Jinan City, Shandong Province in 2011.
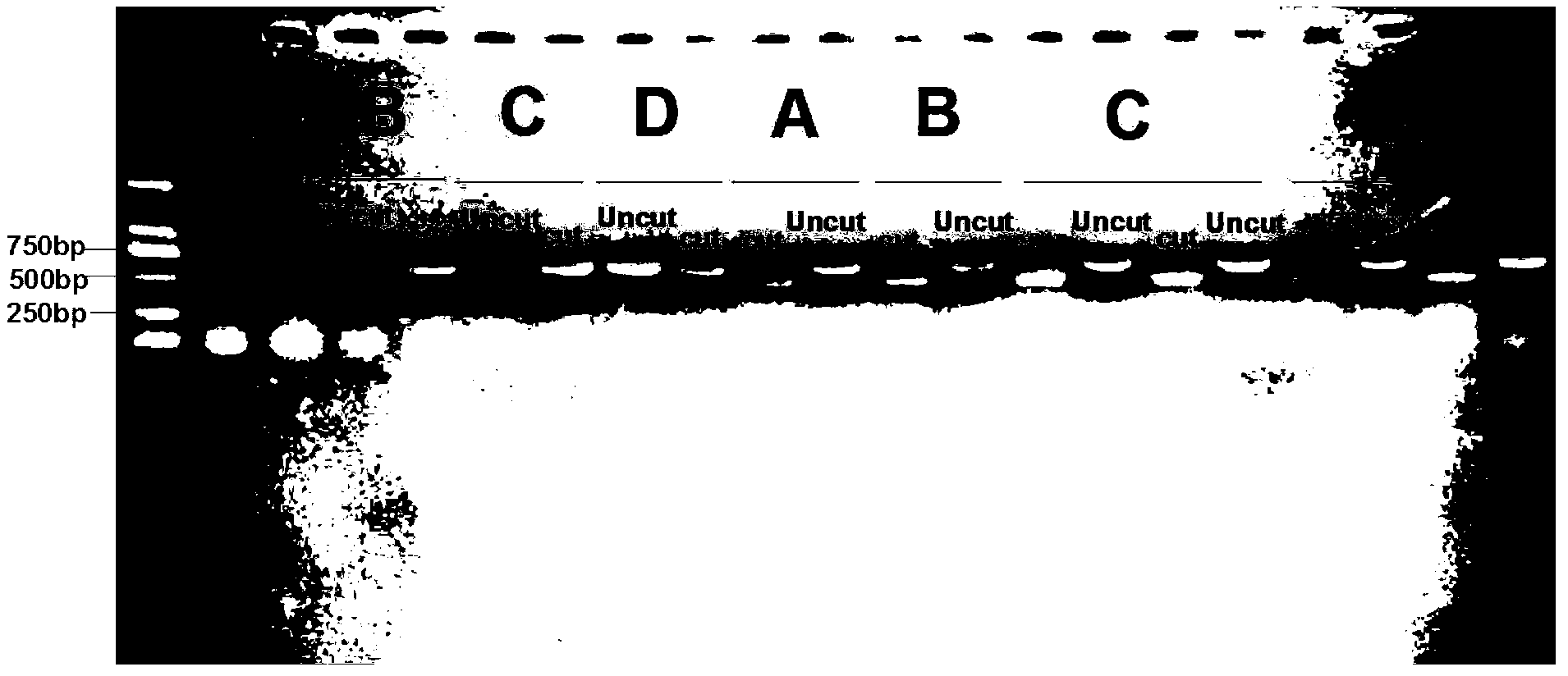
[0064] The results show that when there are two bands of about 200bp and 400bp on the imaging film, it is a leek maggot; when there is a band with a fragment length of about 600bp on the imaging film, it is a maggot, and the results are as follows figure 1 As shown, and in accordance with (Shi Baocai, Lu Hong, Gong Yajun, etc., 2010. Identification and control of Chive tardigrades. Chinese Vegetables, 11:21-22.; Zhang Hongrui, Zhang Xiaoyun, Shen Dengrong, etc., 2008. Edible mushrooms Biological characteristics of the eye fungus Bradysia difformis. Chinese Edible Fungi, 27 (6): 54-56.) The detection results are consistent with the method recorded.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com