Quinazoline derivative and application thereof in organic electroluminescent device
A technology of electroluminescent devices and derivatives, which is applied in the field of white light lighting, can solve the problems of preparation process limitations and limited applications, and achieve the effect of good spectral stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: the synthesis of intermediate
[0017] In a 500 mL three-necked flask, add 100 mL (100 mmol) of a 1 M tetrahydrofuran solution of phenylmagnesium bromide, add 100 mL of dry diethyl ether, and heat to reflux in an oil bath at 45°C. A solution of 5.91 g (50 mmol) of 2-cyanoaniline in 50 mL of dry ether was added dropwise thereto over 30 minutes. After further reflux for 1.5 hours, it was cooled to 0°C in an ice-water bath. Then, a solution of 13.2 g (60 mmol) of 4-bromobenzoyl chloride in 100 mL of dry ether was added dropwise, and heated to reflux in an oil bath at 45° C. for 2 hours. After the reaction was completed, the mixture was cooled to 0° C. in an ice-water bath, and saturated aqueous ammonium chloride solution was added. The precipitate was taken out by filtration, washed with a small amount of methanol, and then vacuum-dried to obtain 7.59 g of an intermediate (yield 42%).
Embodiment 2
[0018] Embodiment 2: the synthesis of compound (1) and (2)
[0019] Under nitrogen conditions, add palladium acetate 25mg and tri-tert-butylphosphine 0.1 mL, heated to reflux for 4 hours. After stopping the reaction, the solvent was removed, and the residual substance was separated on a silica gel column with dichloromethane as the eluent to obtain compound (1). 1H NMR (300MHz; CDCl 3 ):δ8.93(d,J=8.4Hz,2H),8.27(d,J=8.7Hz,1H),8.18(m,3H),7.91–7.98(m,3H),7.74–7.78(m, 2H),7.59–7.66(m,4H),7.54(d,J=8.4Hz,2H),7.41–7.47(m,2H),7.28–7.34(m,2H).
[0020] The synthesis of compound (2) is the same as that of compound (1), just replace carbazole with diphenylamine. 1H NMR (300MHz; CDCl 3 ):δ8.54(d,J=7.8Hz,2H),8.12(d,J=8.7Hz,2H),7.85–7.89(m,3H),7.57–7.60(m,3H),7.50–7.55( m,1H),7.25-7.31(m,4H),7.18(d,J=8.7Hz,6H),7.05–7.10(m,2H).
Embodiment 3
[0021] Example 3: Light-emitting device [ITO / NPB / TCTA / (1) / TPBI / LiF / Al]
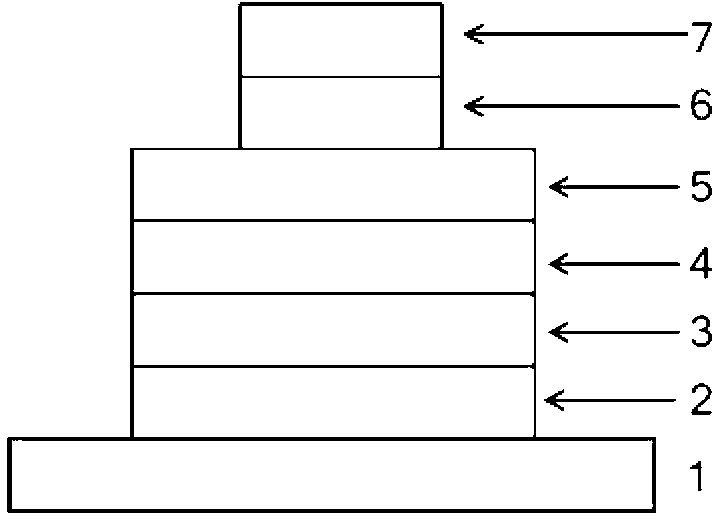
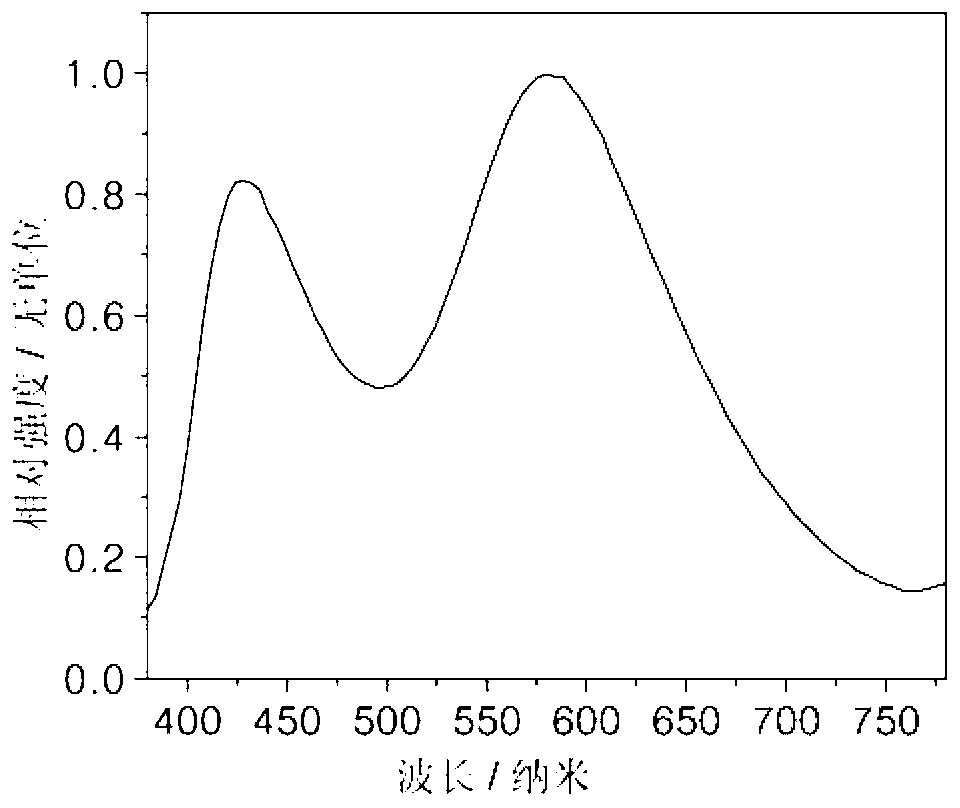
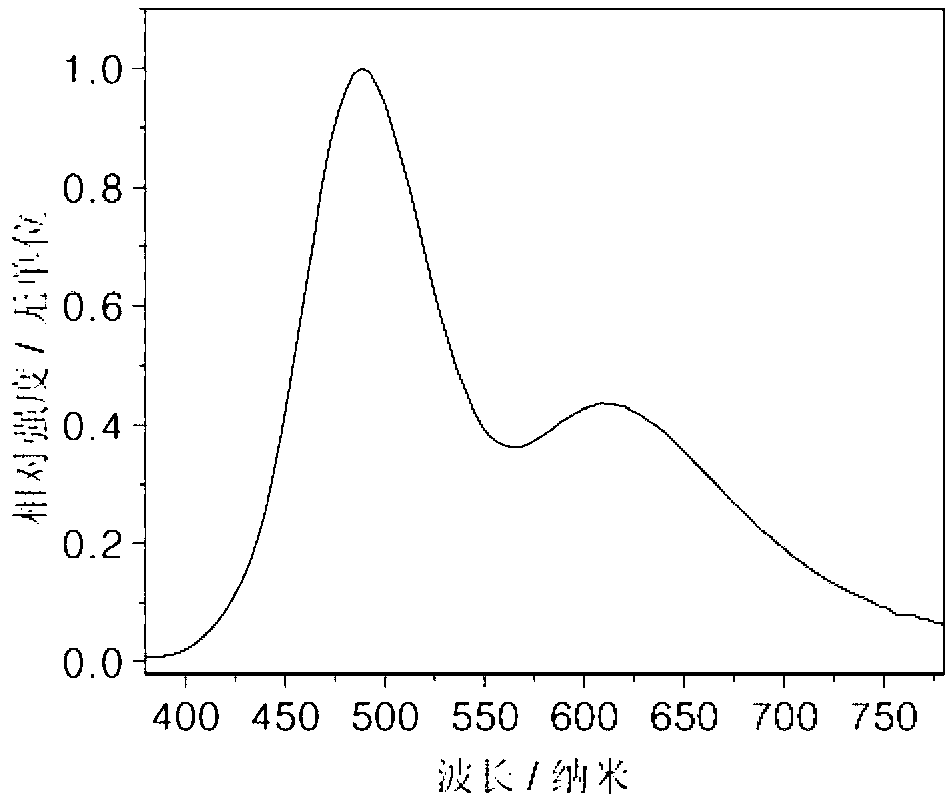
[0022] On the glass substrate coated with ITO anode, the hole transport layer NPB (40nm), the electron blocking layer TCTA (5nm), the compound (1) is the light emitting layer (15nm), the electron transport layer TPBI (45nm), and the electron injection Layer LiF (1nm), Al cathode (100nm). Maintain a pressure of 5×10 during the evaporation process -6 Pa. The fabricated device has a turn-on voltage of 3.2V and a maximum brightness of 1945cd m -2 , the maximum current efficiency is 0.93cd A -1 , the maximum energy efficiency is 0.88lm W -1 , the luminous peak is at 445nm, which is blue light emission.
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com