A Cu-Zn-based catalyst for reverse water gas shift reaction, its preparation method and application
A technology for shift reaction and reverse water gas, which is applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc. The preparation method and application patent report of the shift reaction catalyst have achieved the effect of good industrial application prospects, low requirements for reaction equipment and stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
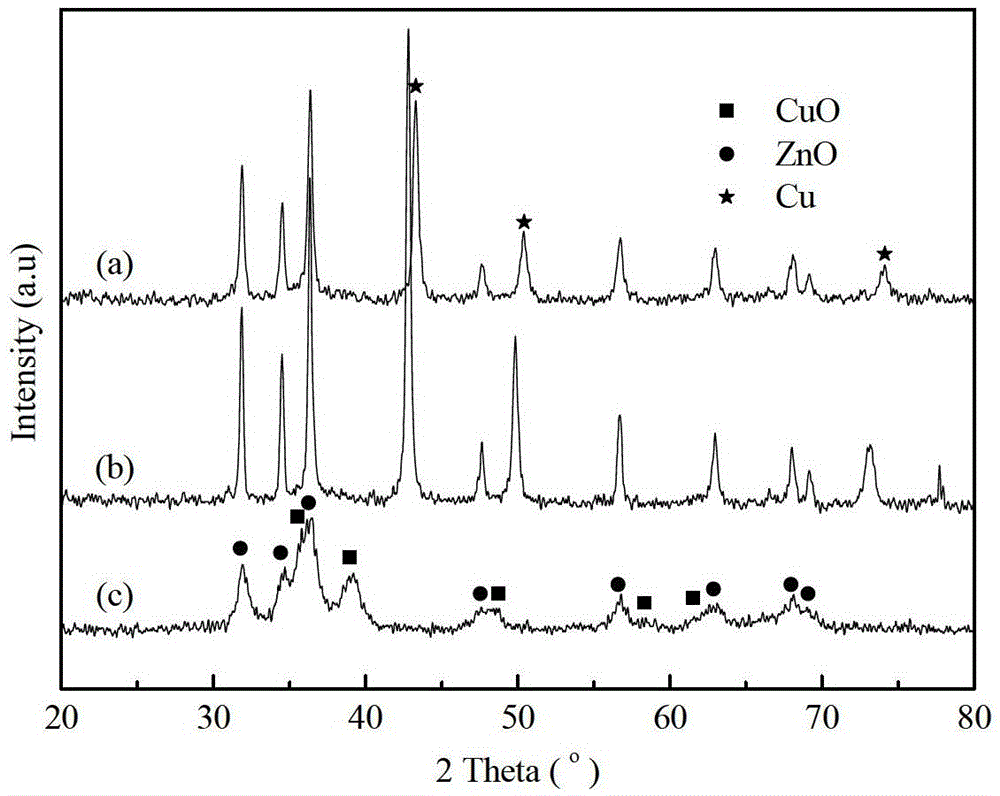
Image
Examples
Embodiment 1
[0039] 1. Catalyst Cu 46.5 Zn 46.5 K 7 preparation of
[0040] 1) Prepare 0.5mol / L Cu and Zn nitrate aqueous solutions respectively, and mix them according to the mole fraction ratio of Cu and Zn in the catalyst; mix the above mixed aqueous solution with the prepared 0.5mol / L Na 2 CO 3 Aqueous solution, under the action of continuous stirring at 70 ℃, add dropwise to carry out co-precipitation; maintain the pH of the precipitation slurry = 8.0, after the precipitation is completed, raise the temperature to 80 ℃, continue to stir for 30 minutes; then wash at room temperature for 1 hour Filter to free of Na + , to obtain a Cu-Zn-based carrier;
[0041] 2) Using the equal volume impregnation method, configure 0.5mol / L K 2 CO 3 Aqueous solution, according to the ratio of the above expression (Cu 46.5 Zn 46.5 K 7 ), using the solution to impregnate the Cu-Zn-based carrier obtained in step 1) for 8h to obtain a catalyst precursor;
[0042] 3) Put the obtained catalyst pre...
Embodiment 2
[0051] Reference Example 1 Catalyst Cu 46.5 Zn 46.5 K 7 Preparation, by the expression Cu 50 Zn 40 Na 7 Mg 3 Prepare the catalyst, configure the acetates of Cu and Zn into 1.5mol / L aqueous solutions respectively, and mix them, and mix the above-mentioned mixed aqueous solutions with the configured 1.5mol / L Na 2 CO 3 The aqueous solution was added dropwise to carry out co-precipitation, and the mixed aqueous solution of the nitrate configuration of 1.0mol / LNa and Mg was used to replace the 0.5mol / L K 2 CO 3 aqueous solution.
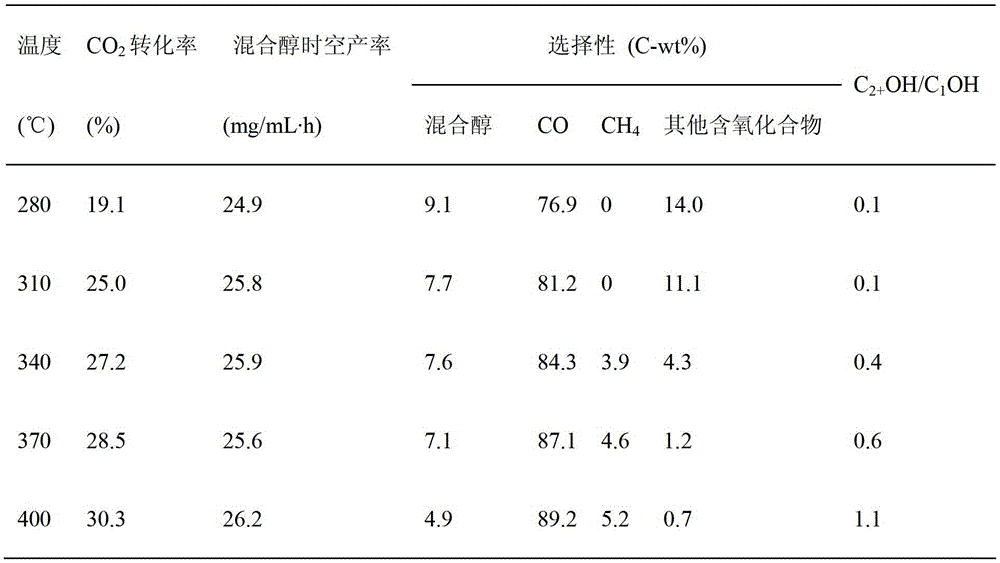
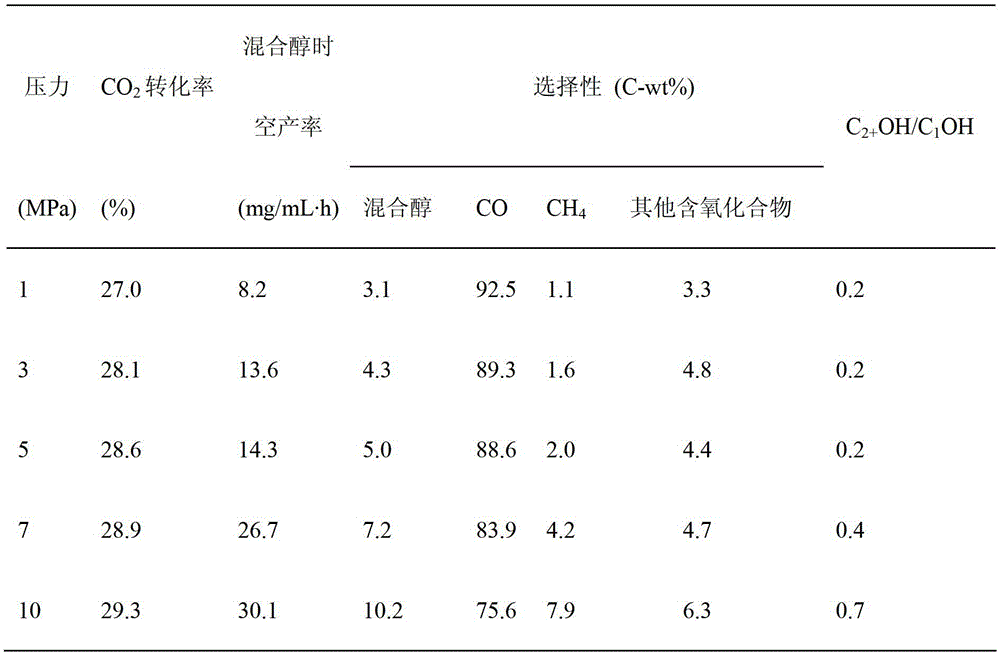
[0052] In Cu 50 Zn 40 Na 7 Mg 3 Catalyst is applied in reverse water gas shift reaction process, and catalyst loading and reduction activation condition are identical with embodiment 1; Reaction condition is: H 2 / CO 2 The molar ratio is 3, 340°C, 1-10MPa, volume space velocity 5000h -1 Catalyst evaluation was carried out below, and the obtained results are shown in Table 2.
[0053] Table 2
[0054]
Embodiment 3
[0056] Reference Example 1 Catalyst Cu 46.5 Zn 46.5 K 7 Preparation, by the expression Cu 60 Zn 30 K 10To prepare the catalyst, configure the nitrates of Cu and Zn into 3.0mol / L aqueous solutions respectively, and mix them, and add the above-mentioned mixed aqueous solution and the configured 3.0mol / L KOH aqueous solution in parallel for co-precipitation; 2.0mol / L of KNO was used in the impregnation preparation process of the catalyst 3 Aqueous solution replaces the K of 0.5mol / L among the embodiment 1 2 CO 3 aqueous solution.
[0057] In Cu 60 Zn 30 K 10 The catalyst is used in the reverse water gas shift reaction process, and the catalyst loading is the same as that in Example 1; the catalyst is reduced and activated except that 10% H 2 / N 2 The mixed gas is except reducing gas, and other is identical with embodiment 1; Reaction condition is: H 2 / CO 2 The molar ratio is 3, 340°C, 5MPa, and the volume space velocity is 1000~10000h -1 Catalyst evaluation was ca...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com