Screening kit for seventh chromosome abnormality diseases of fetus
A chromosomal abnormality and kit technology, applied in the field of kits, can solve problems such as unbalanced gametes, and achieve the effects of simple operation, high patient compliance, and good application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 sample collection
[0019] 1. Collection object: A total of 35 pregnant women in the first trimester visited the outpatient clinic of West China Second Hospital of Sichuan University and the Second Affiliated Hospital of Chengdu University of Traditional Chinese Medicine from October 2011 to April 2012.
[0020] 2. Diagnostic criteria for early pregnancy: refer to the method recorded in the 7th edition of the textbook "Obstetrics and Gynecology" of national higher medical colleges (including ① history of menopause; ② HCG positive; gestational sac).
[0021] 3. Inclusion criteria: ① Those who meet the above diagnostic criteria; ② Gestational age: 5 weeks to 13+6 weeks; ③ Informed consent, voluntary subjects.
[0022] 4. Exclusion criteria: patients with cardiovascular, liver, kidney and hematopoietic system complications, or other primary diseases and mental illness.
[0023] 5. Drop-out criteria: ① Cases that do not meet the inclusion criteria or are accompa...
Embodiment 2
[0029] The detection method of embodiment 2PLGF level
[0030] 1. Main instruments
[0031] (1) Microplate reader: Molecular Devices, Spectra Max5
[0032] (2) Refrigerated centrifuge: BECKMAN company, AllegraTM64R
[0033] (3) -20°C Refrigerator: Electrolux Company
[0034] (4) Ultra-low temperature refrigerator: Thermo company, SK501
[0035] (5) Electric constant temperature incubator: Shanghai Senxin Experimental Instrument Co., Ltd., DRP-9162
[0036] 2. Experimental reagents and materials
[0037] (1) Capture Antibody: mouse anti-human PLGF monoclonal antibody Human PLGF Antibody (Monoclonal Mouse IgG1), purchased from R&D Company, MAB264;
[0038] (2) Detection Antibody: Biotin-labeled goat anti-human PLGF polyclonal antibody HumanPLGF Biotinylated Antibody (Ployclonal Goat IgG), purchased from R&D Company, BAF264;
[0039] (3) PLGF standard product: recombinant human PLGF Recombinant Human PLGF, purchased from R&D Company, 264-PG-10;
[0040] (4) Streptavidin-ho...
Embodiment 3
[0060] Example 3 Correlation between PLGF level and fetal chromosome 7 abnormality
[0061] The blood and urine samples collected in Example 1 were detected respectively according to the method of Example 2, and the statistical results are shown in Tables 1-2:
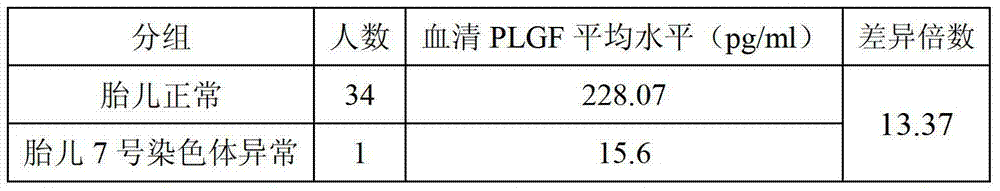
[0062] Table 1 Correlation between fetal chromosome 7 abnormality and serum PLGF level
[0063]
[0064] As shown in Table 1, when the fetus is normal, the serum PLGF of the pregnant woman is 228.07pg / ml, and when the fetal chromosome 7 is abnormal, the serum PLGF of the pregnant woman is as low as 15.6pg / ml, the former is 212.47pg / ml higher than the latter, which is the 14.62 times.
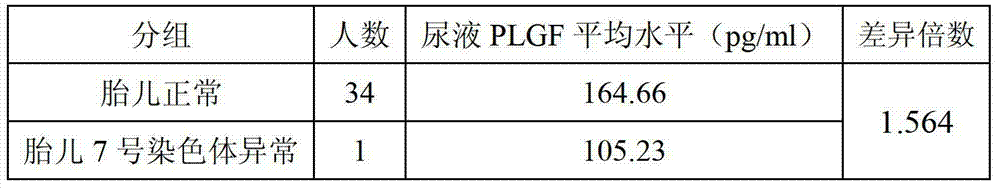
[0065] Table 2 Correlation between fetal chromosome 7 abnormality and urinary PLGF level
[0066]
[0067] As shown in Table 2, when the fetus is normal, the urine PLGF of the pregnant woman is 164.66pg / ml, and when the fetus has abnormal chromosome 7, the urine PLGF of the pregnant woman is 105.23pg / ml, the former is 59.43pg / ml ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com