Cyclizing metal iridium complex and application thereof
A complex, metal iridium technology, applied in the field of transition metal luminescent materials and bioanalysis, can solve the problem of rarely reported pH sensors of iridium complexes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
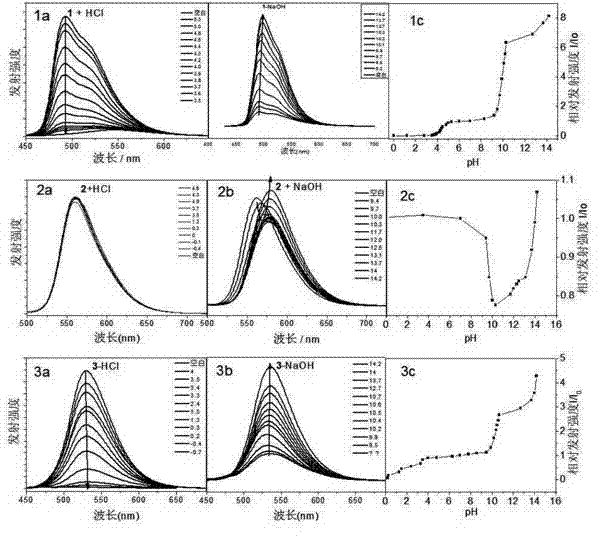
[0042] Iridium complexes containing hydroxyl ligands are used as "OFF-ON" fluorescent sensors in solution: samples are accurately weighed on a balance to make approximately 1x10 -3 mol / L DMSO solution, respectively dilute the sample solution to 6x10 -6 mol / L DMSO / H 2 O (1 / 1, v / v) mixed solution was used to test its luminescence spectrum in pH 0-14 solution. When HCl is added to the DMSO / H of these complexes 2 O (v / v, 1 / 1) solution, the pH of the solution changed from 7-0, the fluorescence of complexes 1 and 3 decreased significantly, but complex 2 did not change significantly. When NaOH changed the pH of these solutions from 7 to above 14, the complexes showed the opposite phenomenon, the fluorescence of complexes 1 and 3 recovered 9-fold and 5-fold and the emission peaks were red-shifted by 9nm and 4nm, respectively. Under alkaline conditions, compound 2 showed a special phenomenon. When the concentration of NaOH was less than 30 times, the fluorescence gradually decreased w...
Embodiment 2
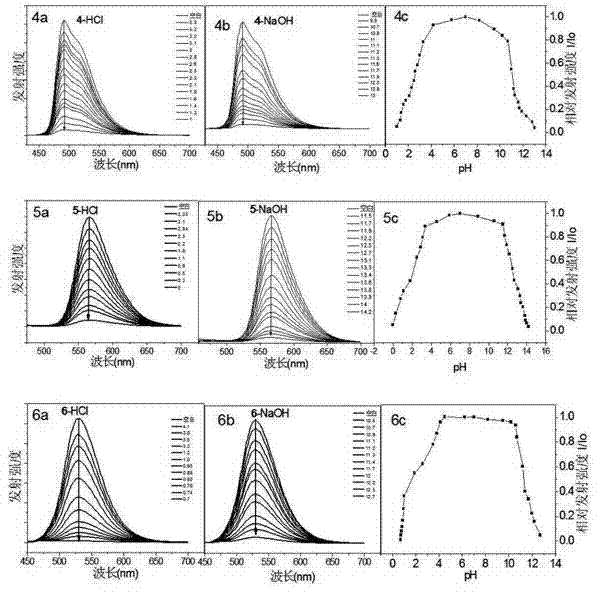
[0044] Iridium complexes containing amino ligands are used as "OFF-ON-OFF" fluorescent sensors in solution: samples are accurately weighed on a balance to make approximately 1x10 -3 mol / L DMSO solution, respectively dilute the sample solution to 6x10 -6 mol / L DMSO / H 2 O (1 / 1, v / v) mixed solution was used to test its luminescence spectrum in pH 0-14 solution. With HCl adjusting the pH from 7.5-0, the fluorescence intensity of compounds 4-6 decreased to almost 0 ( figure 2 ). The difference from the corresponding dihydroxy compounds is that when NaOH was added to the solutions of compounds 4, 5, and 6, the luminescence of these compounds decreased significantly, respectively. The reversibility study found that after six cycles, the degree of fluorescence weakening of the iridium complex containing amino ligands was reduced, and the stability was not as good as that of the dihydroxy complex. From pH-I / I 0 It can be seen from the figure that there is a platform in the reacti...
Embodiment 3
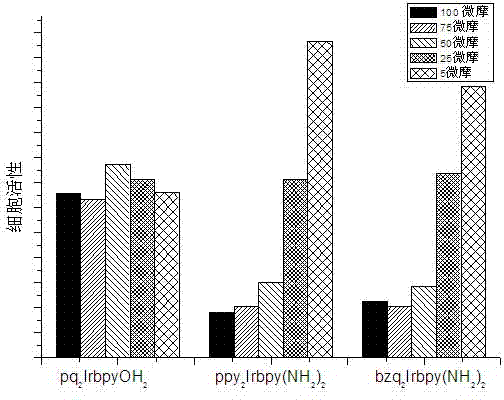
[0046] Cell imaging and toxicity experiment of some cyclized iridium complexes containing hydroxyl ligands and amino ligands: MTT method was used to test the activity of HepG2 cells treated with iridium complexes. HepG2 cells were grown in 96-well tissue culture plates at a density of 4×10 6 cells / well, cultivated for three days, treated with iridium complex for 24 hours, washed the culture plate twice with culture medium, then added MTT, cultured for another 4 hours, and used cells not treated with iridium complex as control. Relative cytotoxicity with the formula [OD sample -OD blank ] / [OD reference -OD blank ] × 100 (OD is the optical density) calculated as a percentage expressed. Each experiment was repeated three times. The complex used in the experiment is [(pq) 2 Ir(bpyOH 2 )] Cl, [(ppy) 2 Ir(bpy(NH 2 ) 2 )]Cl and [(bzq) 2 Ir(bpy(NH 2 ) 2 )] Cl. The experiment found that [(pq) 2 Ir(bpyOH 2 )] Cl, [(ppy) 2 Ir(bpy(NH 2 ) 2 )]Cl and [(bzq) 2 Ir(bpy(NH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com